2351
Diaschisis of The Language Network in Resting State fMRI Functional Connectivity of Post-Stroke Chronic Aphasia1Human Magnetic Resonance Center, University of Massachusetts Amherst, Amherst, MA, United States, 2Department of Communication Disorders, University of Massachusetts Amherst, Amherst, MA, United States
Synopsis
Functional connectivity (FC) of intrinsic networks was compared between two groups: healthy controls and post-stroke aphasia, using resting state fMRI. While the FC of auditory, motor, and default mode networks were preserved, FC of the language network was disrupted in the aphasia group. The aphasia group showed left ipsilateral frontal FC from the Broca area but not from the Wernicke area. Similarly, the aphasia group showed left ipsilateral temporo-parietal FC from the Wernicke area but not from the Broca area. Thus, a clear picture of diaschisis, not just structural disruption, was revealed in the FC of the aphasia group.
Introduction
Resting state functional connectivity (FC) is recognized as an emerging method to study the language network in post-stroke aphasia due to its simplicity in data collection, reproducibility, and integrative analysis of the whole brain network independent of the network distance.1,2,3 Using FC, it was reported that the inter-hemispheric connection was altered more than the inner-hemispheric connection in the language network of semi-acute stroke patients.4 We investigated the alteration of FC of the language network in post-stroke chronic aphasia individuals compared to healthy control subjects.Methods
Resting state fMRI data were collected from 17 healthy and 5 aphasia participants (3 of them were scanned twice at pre and post aphasia therapy), so total 8 entries for the group analysis) at 3T MRI with a 20-ch head-and-neck RF coil. The images were preprocessed with motion correction, spatial smoothing (5mm), and temporal band pass filtering between 0.01 and 0.1 Hz using FSL. The temporal time series of each ROI (sphere of 6 mm radius) was extracted and used as a regressor in the subsequent general linear model (GLM) analysis using FSL’s FEAT for the ROI to the whole brain FC measurement.5 The global signal of the gray matter was also extracted and used as a regressor to remove the physiology noise.6 Each group was averaged using the higher-level analysis with FLAME1 and a cluster p threshold = 0.001 in FSL’s FEAT. The ROI seeds were defined for the language network (bilateral Broca and Wernicke areas)2 and other areas such as left auditory cortex, left motor area, dorsal anterior cingulate cortex (dACC), and posterior cingulate cortex (PCC).Results
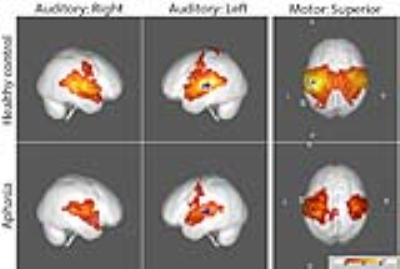
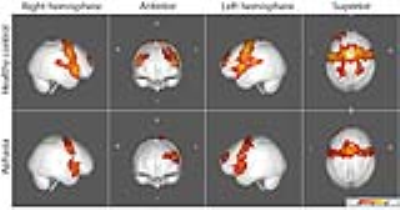
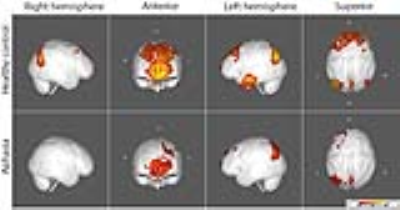
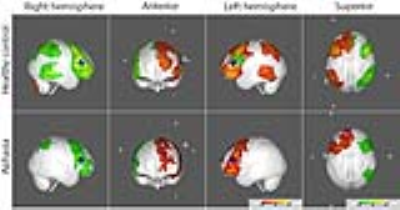
FC maps of the language network and other seed ROI’s shown in the large scale FC study2 and other reported FC reports3 were replicated in our healthy control group, as demonstrated in all the figures. The FC maps for the auditory and motor cortices were similar between the control and aphasia groups (see Fig. 1). The two resting state FC maps from the seeds at dACC and PCC indicated that the overall FC was reduced in the aphasia group as shown in Fig. 2 and Fig. 3, respectively. The reduced statistical power in the aphasia group was expected due to the limited number of the aphasia group. FC using the Broca seed in both hemispheres demonstrated significant disruption to ipsilateral Wernicke and posterior temporal cortex (Fig. 4). FC using the Wernicke seed in the left hemisphere demonstrated significant disruption to the ipsilateral eloquent cortex, including anterior temporal, prefrontal, and left inferior frontal cortices (Fig. 5). Inter-hemispheric FC was found from the right Broca or Wernicke areas to the respective contralateral areas in the control group, but this was disrupted in the aphasia group (Fig. 4 and Fig. 5).Discussions
Fig. 4 and Fig. 5 very clearly demonstrate the principle of diaschisis, i.e., that disruption of FC in aphasia can occur distant from the site of lesion, over and above structural disconnection due to lesion of cortical regions and/or white matter tracts. This is observable in the aphasia group which shows the left ipsilateral frontal FC in Fig. 4 but not Fig. 5; similarly, the aphasia group shows the left ipsilateral temporo-parietal FC in Fig. 5 but not Fig. 4.Conclusion
While FC of auditory, motor, and default mode network was preserved, FC of the language network was disrupted in the aphasia group. The aphasia group shows left ipsilateral frontal FC from the Broca area but not from Wernicke area. Similarly, the aphasia group shows left ipsilateral temporo-parietal FC from the Wernicke area but not from the Broca area. Inter-hemispheric disruption of the language network in the aphasia group was also confirmed. So a clear picture of diaschisis, not just structural disruption, was revealed in FC of the aphasia group.Acknowledgements
This work was supported by a Human MR Center Pilot Grant Program award from the University of Massachusetts Amherst, Institute for Applied Life Sciences to Dr. Kurland.References
1. Klingbeil J, et al. Resting-state functional connectivity: An emerging method for the study of language networks in post-stroke aphasia. Brain Cogn 2017.
2. Tomasi D, Volkow ND. Resting functional connectivity of language networks: characterization and reproducibility. Mol Psychiatry 2012;17(8):841-854.
3. Song X, et al. Data-Driven and Predefined ROI-Based Quantification of Long-Term Resting-State fMRI Reproducibility. Brain Connect 2016;6(2):136-151.
4. Siegel JS, et al. Disruptions of network connectivity predict impairment in multiple behavioral domains after stroke. Proceedings of the National Academy of Sciences of the United States of America 2016;113(30):E4367-4376.
5. Birn RM, et al. The respiration response function: the temporal dynamics of fMRI signal fluctuations related to changes in respiration. Neuroimage 2008;40(2):644-654.
6. Vahdat S, et al. Functionally specific changes in resting-state sensorimotor networks after motor learning. The Journal of neuroscience : the official journal of the Society for Neuroscience 2011;31(47):16907-16915.
Figures