2349
Sleep Quality and Its Impact on Functional Connectivity and Cognitive Performance in HIV Infected Individuals1Electrical and Computer Engineering, University of Rochester, Rochester, NY, United States, 2Dept of Biostatistics and Computational Biology, University of Rochester, Rochester, NY, United States, 3Department of Imaging Sciences, University of Rochester, Rochester, NY, United States, 4Department of Medicine, University of Rochester, Rochester, NY, United States, 5Department of Neurology, University of Rochester, Rochester, NY, United States
Synopsis
We investigated sleep quality in HIV infected individuals and its potential impact on cognitive performance and functional connectivity. Sleep quality was assessed using a self-report questionnaire, Pittsburgh Sleep Quality Index (PSQI). Cognitive performance was measured by a standard battery of neuropsychological tests assessing six cognitive domains, while functional connectivity was assessed by resting-state fMRI. We used a seed-based method to investigate the activation changes associated with the thalamus and frontoparietal network. We found a strong interaction between HIV infection and sleep quality, in the inferior temporal gyrus and the inferior parietal lobule but no deleterious effect on cognitive performance.
Introduction
Sleep abnormalities are one of the most frequent complaints reported by HIV infected individuals1. Sleep deprivation has been reported to affect brain regions and networks associated with attention and working memory (frontoparietal network, FPN), arousal (thalamus), and the default mode network (DMN)2. Functional connectivity (FC) between the thalamus and cortical regions tends to decrease following loss of sleep. Since HIV infection can also affect cortical and subcortical gray matter and white matter3,4, including areas possibly regulating sleep function, we hypothesize that HIV infected individuals would be at greater risk of sleep abnormalities.Method
Twenty-eight HIV-infected individuals (Age 36±13.9 yrs, 26 male(93%) , CD4=589.39 cells/uL, Viral load=105.89 copies/mL) and seventy-eight HIV- controls (Age: 44±13.3, 47 male(60%)) were enrolled. Subjects with HIV dementia, confounding CNS disorders and history of CNS infections other than HIV, were excluded. All subjects underwent sleep quality assessment using a self-report questionnaire, Pittsburgh Sleep Quality Index (PSQI)4. A lower PSQI represents better sleep quality. We used a cut-off score PSQI>5 to define the poor sleep group, while those whose PSQI ≤ 5 were defined as good sleep group (10 out of 28 (35.7%) in HIV-infected, 31 out of 78 (39.7%) in HIV-negative). Cognitive performance was measured by a standard battery of neuropsychological tests assessing six cognitive domains. A total summary Z-score was derived from the six cognitive domains.
MRI data was acquired on a 3T Siemens MAGNETOM Trio MRI scanner equipped with a 32-channel head coil. T1-weighted images were acquired with a 3D MPRAGE(TR/ TI/ TE = 2530/1100/3.44 ms, voxel size = 1x1x1 mm3, flip angle (FA)= 78°, bandwidth = 190 Hz/pixel). Resting-state functional MRI was acquired using a GE-EPI sequence (TR/TE = 2000/30 ms, FA = 90°, voxel size = 4x4x4 mm3; matrix size = 64x64, 30 axial slices, volumes = 150). During the entire 5 minutes resting-state MRI scan participants were instructed to keep their eyes open and avoid falling asleep. For resting-state fMRI processing, the first six volumes were discarded to allow for stabilization of the magnetization. Standard pre-processing steps were performed using Data Processing Assistant for Resting-State fMRI (DPARSF)6 and SPM12, including slice timing correction, head motion correction, co-registration to T1-weighted, normalization, and spatial smoothing with Gaussian kernel (FWHM= 4mm), detrend, and band-pass filtering (0.01Hz to 0.08Hz). Nuisance covariates were regressed out including 6 head motion parameters, signals from white matter and cerebrospinal fluid(CSF).
After pre-processing, FPN was found using Group ICA of fMRI Toolbox (GIFT, http://mialab.mrn.org/software/gift/index.html). Seed regions were selected include thalamus from AAL templates, and FPN. The averaged time course in these regions were used to calculate voxel-wise whole brain functional connectivity (FC). The Fisher z-transformed correlation maps were fed into the 2-way ANOVA to examine the factor of HIV-infection and sleep quality. Subjects’ age was controlled as covariates. The regions significantly affected by [HIV-infection x sleep quality] were identified from the Fmap. We used AlphaSim correction for multiple comparisons, voxel p<.005 combined with cluster size > 30 voxels, yielding p<.01. The surviving brain regions were used as masks to extract the FC for further analysis.
Results
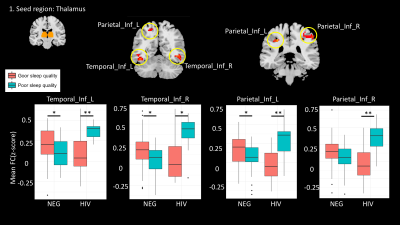
We found significant interaction effect [HIV x sleep quality] on FC between four brain regions and thalamus: inferior parietal lobe left (IPL_L: Number of voxels (#voxels)=69, Peak MNI coordinate (MNI)=-30/-36/42, β=0.420, p<0.001) and right (IPL_R: #voxels=67, MNI=54/-33/48, β=0.428, p<0.001), inferior temporal gyrus left (ITG_L: #voxels 50, MNI=-42/-54/-12, β=0.387, p<0.001), and right (ITG_R: #voxels=43, MNI=45/-57/-6, β=0.408, p<0.001).
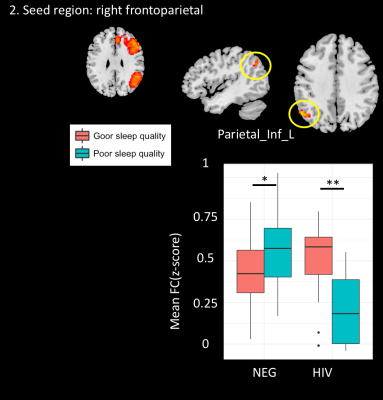
We also found significant interaction effect [HIV x sleep quality] on FC between IPL_L and right FPN (#voxels=37, MNI=-54/-63/42, β=-0.420, p<0.001). No significant interaction effect was found when left FPN was used as a seed.
Within the HIV-uninfected subjects, the FC between thalamus and IPL and ITG were lower in the poor sleep quality group as expected. In addition, the NPS z-scores in HIV-infected subjects were not significantly different between those with good and poor sleep quality.
Discussion
Our results suggest a discordance in functional connectivity between HIV infected and HIV uninfected individuals with poor sleep quality. Specifically, we found increased functional connectivity in areas associated with the thalamus in HIV infected individuals while the trend was in the opposite direction in HIV uninfected controls. One possible explanation is that the underlying substrate for decreased sleep quality is different in HIV infected individuals compared to HIV uninfected, more closely resembling the hyperarousal effect reported in patients with insomnia7.Conclusion
Poor sleep quality did not worsen cognitive performance in HIV infected individuals. The pattern of functional connectivity in HIV infected individuals suggest hyperarousal.Acknowledgements
No acknowledgement found.References
1. daCosta DiBonaventura, M., et al., The association of HIV/AIDS treatment side effects with health status, work productivity, and resource use. AIDS Care, 2012. 24(6): p. 744-55.
2. Krause, A.J., et al., The sleep-deprived human brain. Nat Rev Neurosci, 2017. 18(7): p. 404-418.
3. Castillo, D., et al., Altered Associations between Pain Symptoms and Brain Morphometry in the Pain Matrix of HIV-Seropositive Individuals. J Neuroimmune Pharmacol, 2017.
4. Zhuang, Y., et al., Combination antiretroviral therapy improves cognitive performance and functional connectivity in treatment-naive HIV-infected individuals. J Neurovirol, 2017. 23(5): p. 704-712.
5. Buysse, D.J., et al., The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res, 1989. 28(2): p. 193-213.
6. Chao-Gan, Y. and Z. Yu-Feng, DPARSF: A MATLAB Toolbox for "Pipeline" Data Analysis of Resting-State fMRI. Front Syst Neurosci, 2010. 4: p. 13.
7. Lee, Y.G., et al., Changes in subcortical resting-state functional connectivity in patients with psychophysiological insomnia after cognitive-behavioral therapy: Changes in resting-state FC after CBT for insomnia patients. Neuroimage Clin, 2018. 17: p. 115-123.
Figures