2347
Changes in Brain Function induced by Chronic Neuropathic Pain in a Mouse Model of Chronic Nerve Constriction Injury1University of Erlangen-Nuremberg, Institute of Pharmacology and Toxicology, Erlangen, Germany
Synopsis
The aim of this study was to investigate the effects of the well-established chronic constriction injury (CCI) model on central nociceptive processing in mice over a period of 56 days. For this purpose two behavioral tests (Hargreaves and electronic pressure-meter test [“plantar test”]) and functional MRI were combined. The ligation of the sciatic nerve induced behavioral changes indicative of a neuropathic pain state. Graph theoretical analysis of functional connectivity revealed known effects of chronic pain for the first time also for the CCI model: modifications of the sensory as well as emotional system induced by thermal but also mechanical stimulation.
Introduction
Neuropathic pain has been described as the “most terrible of all tortures which nerve wound may inflict” [1]. A peripheral nerve injury often leads to the development of persistent neuropathic pain, which is characterized by spontaneous especially burning pain, allodynia and hyperalgesia [2]. Frequent therapeutic options lead to minimization of pain up to 50%, improvement of the quality of sleep and life and preservation of the ability of work and social connections [3]. The increased incidence of this disease, the reduced quality of life and the deficient therapeutic options establish the urgent necessity of intensive research and enhancements of preventative measures and therapeutic opportunities.Methods
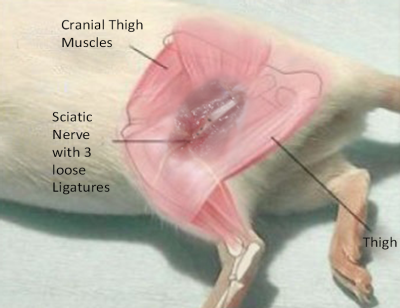
The effects of the well-established chronic constriction injury (CCI) model on central nociceptive processing in mice (C57BL/6; male) were imaged using BOLD fMRI (4.7T Bruker Biospec, 2x2 phased array head coil, single-shot GE EPI sequence ,TEef 25.035ms; TR 4000ms; FOV 15x15mm; matrix 64x64; slice thickness 0.5mm; 22 slices axial, scan duration 75min). In CCI (n=12) 3 ligatures were placed around the sciatic nerve (left side, exposed but not ligated in sham group (n=6) cf. Fig.1). Both hind paws were dorsally stimulated alternately with non-noxious 40°C / 45°C and noxious 50°C / 55°C (this whole set was repeated 3 times) and subsequent local plantar mechanical stimulation (plantar, 6 sets of pneumatically operating von-Frey filaments with 40g). To survey the chronic peripheral neuropathic pain development and its effect on central nociceptive processing, the mice were measured with fMRI on day -1, 4, 6, 8, 14, 21, 28 and 56 post-op, interlaced by repetitive Hargreaves- and von-Frey behavioral tests to verify successful establishment and time-course of the CCI.
Standard preprocessing was performed including interslice time, motion correction as well as spatially and temporally smoothing [4] .For identification of the different brain regions we used a mouse brain atlas based on Paxinos [5]. Besides classical GLM analysis (second order analysis of SPMs and response amplitude) additional graph-theoretical analysis was applied using the same atlas brain regions to detect changes in functional connectivity (FC).
Results
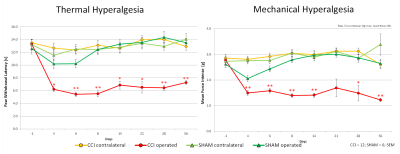
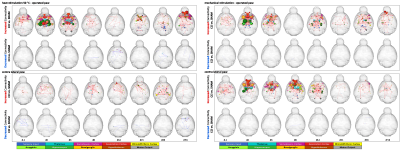
The CCI group showed a consistent decrease of thermal and mechanical paw withdrawal latency (PWL) over the whole 56 days (Fig. 2). Classical BOLD analysis did not reveal significant differences over the repetitive measurements. However, significant changes, dominantly increases, in FC could be shown for many structures of the so-called ‘pain matrix’ (Fig 3). When clear noxious temperature of 50°C was applied to the injured paw distinct effects were visible mainly between days 4 to 6 and as a potentially sign for pain chronification on day 56. In the CCI group compared to the SHAM group, a widespread decrease in FC was found in thalamic, hippocampal and cortical structures like caudate putamen, sensory and motor cortex at days 4, 6 and 56 (operated paw; 50°C). These findings fit nicely the disease activity peak reflected by PWL, which also peaked between days 6 and 8. The ipsilateral paw showed a similar biphasic development of changes in FC. Comparable changes in connections were found for thermal and mechanical stimulation (cf. Fig. 3).Conclusions
The present study examined the time-course of changes in brain function induced by chronic neuropathic pain. Graph-theoretical analysis of FC was much more sensitive to changes in brain function as the classical BOLD analysis.
We could detect early (subchronic; days 4 to 6) but also late (chronic; day 56) functional connectivity changes between areas which are important for emotional-affective (thalamus, hippocampus and cingular cortex) as well as for sensory-descriptive component of nociception (caudate putamen, sensory and motor cortex).
We propose that a chronic, here neuropathic trauma, changes profoundly the processing of nociceptive signals in the brain in addition to the much better known peripheral effects. Studying nociception in mice non-invasively by fMRI can help to understand the transition in central processing from acute to chronic (neuropathic) pain thereby providing (translational) options to improve the therapy for chronic pain patients in the future.
Acknowledgements
BMBF Neurorad (02NUK034D)References
[1] Mitchell S.W. Injuries of nerves and their consequences, JB Lippincott, Philadephia, 1872.
[2] Woolf C.J., Mannion R.J. Neuropathic pain: aetiology, symptoms, neuromechanisms, and management. Lancet (1999) 353 1959–1964.
[3] Finnerup et al. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. The Lancet Neurology, 14(2), 162-173,2015.
Binder, A. and Baron, R. Pharmakotherapie chronischer neuropathischer Schmerzen. Deutsches Ärzteblatt, 113(37), 616-625, 2016
[4] Hess et al. Blockade of TNF-alpha rapidly inhibits pain responses in the central nervous system, Proc Natl Acad Sci U S A, 108 (9), 3731-6, 2011
[5] Franklin KBJ, Paxinos G. The Mouse Brain in stereotaxic coordinates. Academics Press, New York, 3 Ed. 2008
Figures