2344
Preventive anti-NGF treatment suppresses alterations in functional connectivity imposed by cancer-induced bone pain in mice1Inst. for Biomedical Engineering, ETH & University of Zurich, Zürich, Switzerland, 2Neuroscience Center Zurich (ZNZ), Zürich, Switzerland, 3Inst. of Pharmacology & Toxicology, University of Zurich, Zürich, Switzerland
Synopsis
The efficacy of an anti-nerve growth factor (NGF) antibody in preventing rearrangements of whole-brain functional connectivity elicited by nociceptive input following bone metastases was evaluated in a mouse model of cancer-induced bone pain using longitudinal resting-state fMRI. ROI-based network and seed-based connectivity analysis approaches revealed major hubs of ascending and descending pain pathways to be affected by the developing pain. Functional rearrangements within these regions could be prevented by prospective application of anti-NGF antibody mAb911 indicating the efficacy of anti-NGF treatment in preventing, or at least delaying, adaptations of the brain circuitry associated with development of a chronic pain state.
Purpose
Cancer-induced bone pain arises from a primary tumor in the bone or skeletal metastasis of
common cancer types such as breast, lung or prostate cancer [4]. Therapies based on
bisphosphonates, NSAIDs, radiotherapy as well
as centrally active drugs such as opioids are effective in alleviating ongoing pain, but often
exert severe side-effects [6]. Antibodies targeting
nerve growth factor (NGF) have been shown to effectively relieve neuropathic
aspects of cancer-induced bone pain both in mice and humans [2,3,5]. Yet, the impact of NGF inhibition on
the central nervous system remains largely unknown. We therefore investigated
the effects of anti-NGF treatment on functional connectivity in major ascending
and descending pain pathways in a mouse model of cancer-induced bone pain using
resting-sate fMRI (rs-fMRI).Material & Methods
Mouse models of chronic pain from bone cancer were prepared by intramedullar
injection of EO771 (tebu‐bio, Le-Perray-en-Yvelines, France)
breast cancer cells into the tibia of female C57BL/6 (n=8). Functional changes
were assessed longitudinally using a Biospec 94/30 small animal MR
system (Bruker, Karlsruhe, Germany) with a cryogenic quadrature
transmit/receive surface coil for excitation and signal reception. Rs-fMRI data
was acquired using a gradient‐echo echo‐planar imaging (GE‐EPI) sequence: TR=1000ms, TE=12ms, FA=60°, number of averages=1, 900 repetitions. Eighteen consecutive
slices of 0.5mm thickness with an in-plane resolution of 200mmx200mm have been recorded. Animals were anesthetized
using medetomidine hydrochloride (0.05mg/kg as bolus,
0.1mg/kg/h maintenance, i.v.), intubated and ventilated with low dose isoflurane
(0.5%) in 20%O2/80%air mixture at 80breaths/min. For immobilization,
pancuronium bromide was administered s.c. in a bolus of 0.5mg/kg. Behavioral
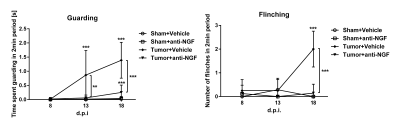
readouts of pain were assessed in terms of time spent guarding and number of
flinches in a 2min period. Murine monoclonal anti-NGF antibody mAb911 (10mg/kg,
i.p., Pfizer Inc.) was administered prospectively. Rs-fMRI data was analyzed
using a ROI-based network analysis and seed-based connectivity. To
test for differences in evolution of functional connectivity between groups a
linear mixed model analysis was used, testing for significant group and session
interactions. Four groups have been included: Tumor bearing animals receiving
either mAb911 or vehicle (‘Tumor+antiNGF’, ‘Tumor+Vehicle’) as well as
sham-operated mice receiving either of the treatments (‘Sham+anti-NGF’, ‘Sham+Vehicle).Results
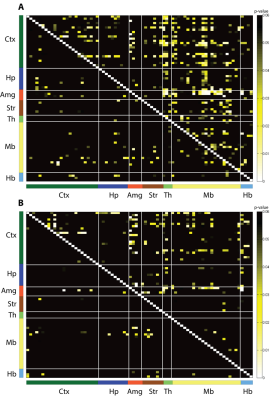
ROI-based analysis of longitudinal rs-fMRI data revealed distinct
rearrangement of functional connectivity (FC) patterns in Tumor+Vehicle as
compared to Sham+Vehicle animals (Fig.2). ROI-based network analysis revealed interactions
of amygdallar nuclei with thalamic as well as midbrain regions to be most
significantly affected. In addition, FCs between amygdallar nuclei and cortical areas were
found to be altered, in particular interactions involving the temporal
associative/insular regions and the cingulate cortex. Furthermore, FC between
the thalamus and cortical areas were specifically affected in somatosensory and temporal associative/insular regions. Less distinct changes were observed
between striatal and midbrain nuclei as well as between midbrain and thalamic
regions. Prospective treatment of tumor-bearing mice using mAb911
successfully prevented these network changes induced by persistent peripheral
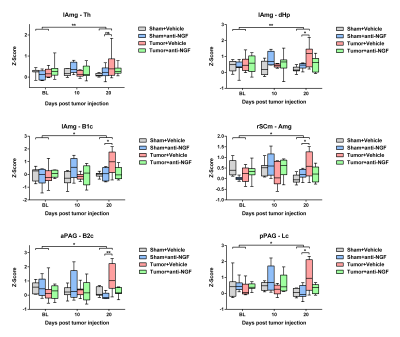
input. Seed-based connectivity analysis revealed significant
effects on FC between the amygdallar seed contralateral to the tumor site and bilateral
thalamic (p=0.004), dorsal hippocampal (p=0.006) and somatosensory (p=0.021) resting
state networks (RSN) (Fig.3). RSNs constitute bilateral group-level independent
components, extracted from naïve mice using independent component analysis
(MELODIC [1]). Furthermore,
FC between the anterior periaqueductal gray seed and barrelfield 2 cortex
(p=0.032), posterior periaqueductal gray seed and limb cortex (p=0.032) as well
as ipsilateral motor related superior collicular seed and the amygdala
(p=0.035) RSNs were found to be significantly affected in the Tumor+Vehicle
group. These changes in FC could be prevented by treatment with anti-NGF
antibodies.Discussion
Our study revealed FC alterations involving the major hubs of ascending
and descending pain pathways in a mouse model of persistent pain from
bone metastasis. We found profound alterations affecting
connections form the contralateral amygdallar nuclei to cortical
somatosensory (B1c, B2c, Lc) and limbic structures such as cingulate, temporal
associative and insular cortex and midbrain. Seed-based analysis
supported these findings and furthermore indicated altered connectivities
between the anterior and posterior periaqueductal gray and cortical structures. Cancer
pain associated FC alterations could be successfully prevented through the
preventive administration of anti-NGF antibody mAb911 with only few residual
interactions. Seed-based analysis indicated no changes in connectivity strength
as a function of time in Tumor+anti-NGF mice as
compared to Sham+Vehicle animals throughout the observation period, indicating
efficacy of anti-NGF treatment in preventing FC rearrangements in response to
cancer-induced bone pain.
Conclusion
Using longitudinal rs-fMRI readouts in a mouse model of cancer-induced
bone pain we were able to show efficacy of anti-NGF treatment in preventing
pain-induced alterations in FC involving major nuclei of ascending and
descending pain pathways. Monitoring the adaptation of the brain circuitry in
such models may yield information on mechanisms contributing to the chronification
of pain during persistent bone cancer-induced nociceptive input.
Acknowledgements
No acknowledgement found.References
[1] Beckmann CF, Smith SM. Probabilistic Independent Component Analysis for Functional Magnetic Resonance Imaging. IEEE Trans. Med. Imaging 2004;23:137–152. doi:10.1109/TMI.2003.822821.
[2] Cattaneo A. Tanezumab, a recombinant humanized mAb against nerve growth factor for the treatment of acute and chronic pain. Curr. Opin. Mol. Ther. 2010;12:94–106. Available: http://www.ncbi.nlm.nih.gov/pubmed/20140821. Accessed 6 Oct 2017.
[3] Jimenez-Andrade JM, Ghilardi JR, Castañeda-Corral G, Kuskowski MA, Mantyh PW. Preventive or late administration of anti-NGF therapy attenuates tumor-induced nerve sprouting, neuroma formation, and cancer pain. Pain 2011;152:2564–2574. doi:10.1016/j.pain.2011.07.020.
[4] Mantyh P. Bone cancer pain: Causes, consequences, and therapeutic opportunities. Pain.2013, Vol. 154.
[5] Sevcik MA, Ghilardi JR, Peters CM, Lindsay TH, Halvorson KG, Jonas BM, Kubota K, Kuskowski MA, Boustany L, Shelton DL, Mantyh PW. Anti-NGF therapy profoundly reduces bone cancer pain and the accompanying increase in markers of peripheral and central sensitization. Pain 2005;115:128–141. doi:10.1016/j.pain.2005.02.022.
[6] World Health Organization. Cancer pain relief : with a guide to opioid availability. World Health Organization, 1996 p.
Figures