2343
Hot-wiring of functional brain connectome in neurologically asymptomatic patients with primary insomnia1Department of Medical Imaging, Guangdong No. 2 Provincial People's Hospital, Guangzhou, China, 2Philips Healthcare, Hong Kong, China, 3Philips Healthcare, Beijing, China
Synopsis
Functional and structural neuroimaging studies have revealed abnormal of Primary Insomnia (PI) patient's brain, including decreased gray matter density, and increase of spontaneous brain activity and metabolism in hippocampus and fronto-parietal cortex, and so on. We use graph-based approaches to investigate the topological abnormalities of functional brain networks in PI patients and examine clinical correlates of the alterations. PI patients exhibited increased overall connectivity of functional brain networks and nodal efficiency in the default mode network (DMN) and emotional circuit. This abnormal organization of large-scale functional brain networks in PI, which could account for memory and emotion dysfunction in PI patients.
Introduction
Primary insomnia (PI) is one of the most prevalent psychiatric syndromes,1,2 and could develop to a series of psychiatric and cognitive disorders (e.g., depressive and anxiety disorders)3,4 and have tremendous negative socio-economic impacts . 5,6 However, the neurobiological mechanisms of PI are not fully understood. Using structural MRI, brain atrophy is consistently observed in PI in a specific set of regions, such as the hippocampus 7,8 and fronto-parietal cortex . 9,10 fMRI and PET studies have reported insomnia related increases in spontaneous brain activity and metabolism in multiple brain regions. 11-15 Besides the localized alterations, more recent studies began to examine abnormal interregional functional integrations in insomnia given the interconnected nature of the human brain.16-18 These studies collectively imply that PI can be viewed as a global rather than a focal disease that affects interregional neural coordination of multiple functional systems. In the present study, we aim to investigate the topological organization of functional brain networks with graph theory analysis in PI patients, and examine clinical correlates of the alterations.Methods
36 PI patients and 38 age-, gender- and education-matched healthy controls (HCs) were recruited in the current study. All participants were scanned using a 3.0T MR scanner (Ingenia; Philips, the Netherlands) and underwent a series of neuropsychological assessments. Resting-state fMRI data acquisition was performed with a BOLD-EPI sequence, anatomical 3D T1 weighted images were acquired for brain ROI delineation. Data preprocessing included realignment, spatial normalization filtering and regression. Then, we parceled the cerebrum into 246 regions of interest (ROIs) based on a prior brain atlas, 19 and calculated the mean BOLD signal time series for each ROI. To remove spurious interregional correlations in the resulting correlation matrices, only correlations with corresponding P-values above a statistical threshold (P < 0.05) were kept nonzero. Finally, we calculated graph based metrics in the context of a weighted network (G) with nodes (N) and edges (K), including global small-world network efficiency, and local nodal centrality. Between-group differences in network properties (global efficiency, local efficiency, normalized global efficiency, normalized local efficiency and nodal efficiency) were inferred by nonparametric permutation tests. Partial correlation analyses were used to assess the relationships between neuropsychological measurements and network metrics.Results
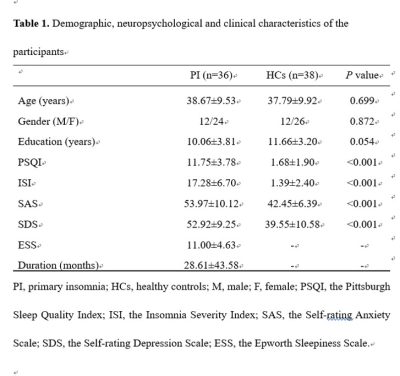
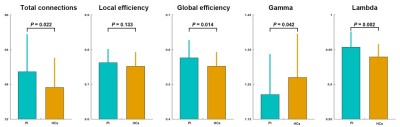
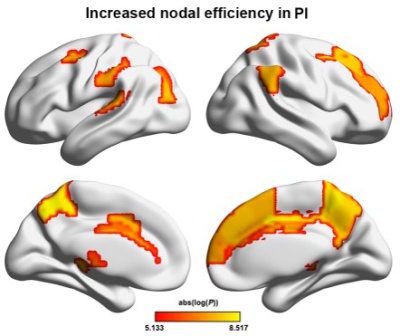
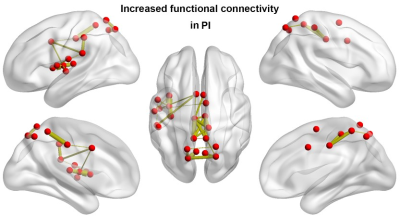
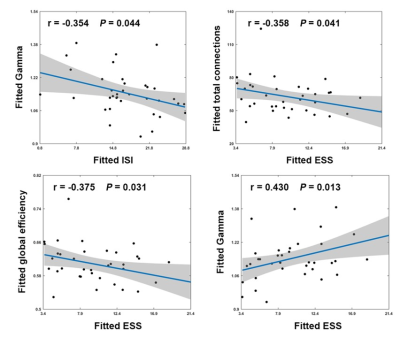
There were no significant between-group differences in age, gender, or education (P > 0.05) (Fig.1). Graph-based analyses revealed that the patients showed significantly increased total number of edges (P = 0.022), global efficiency (P = 0.014), andnormalized global efficiency (Lambda, P = 0.002) and decreased normalized local efficiency (Gamma, P = 0.042) compared with the HCs (Fig.2). Locally, several prefrontal and parietal regions including the superior temporal gyrus and the thalamus exhibited higher nodal efficiency in the patients (P < 0.05, FDR corrected) (Fig.3). Most of these regions also showed increased functional connectivity in the patients (P < 0.05, FDR corrected) (Fig.4). Finally, the altered network efficiency correlated with neuropsychological variables of Epworth Sleepiness Scale (ESS)20 and Insomnia Severity Index (ISI)21 in the patients(Fig.5).Discussion
In the present study, we first found that PI patients exhibited increased overall connectivity of functional brain networks, which could result in more routing paths and therefore more efficient information propagation and exchanges of a network in PI. Interestingly, we found that the increased overall connectivity and altered network efficiency were correlated with behavior disturbances as measured by the ESS and ISI scores, suggesting the topological alterations of functional brain networks could account for excessive daytime sleepiness and sleep dysfunction of the PI patients. At the nodal level, multiple regions showed increased nodal efficiency in the PI patients that were mainly in the DMN and emotional circuit. The DMN includes a set of anatomically and functionally interconnected regions that are engaged in a wide spectrum of cognitive processing. Clinically, working memory deterioration is the most common symptom of daytime dysfunction in PI , 22,23 thus we speculate that the increased nodal efficiency and functional connectivity of the DMN regions may reflect a compensatory mechanism of the patients’ brains to maintain cognitive processing by adding or establishing new connections. Moreover, our findings are consistent with previous findings and provide further evidences for the emotional hyperarousal hypothesis from the perspective of functional integration. 14,24 We speculate that the efficiency increases in emotional regions may underlie emotional dysfunction frequently observed in PI.Conclusion
PI is associated with abnormal organization of large-scale functional brain networks, which could account for memory and emotional dysfunctions in PI patients. Our findings provide direct evidence for network disorganization in PI, which may contribute to a better understanding of the neurobiological mechanisms of PI.Acknowledgements
We thank all the patients and volunteers for participating in this study.References
1. Roth T. Comorbid insomnia: current directions and future challenges. Am J Manag Care. 2009 Feb;15 Suppl:S6-13.
2. Edinger JD, Bonnet MH, Bootzin RR, et al. Derivation of research diagnostic criteria for insomnia: report of an American Academy of Sleep Medicine Work Group. Sleep. 2004; 27: 1567-1596.
3. Baglioni C, Battagliese G, Feige B, et al. Insomnia as a predictor of depression: a meta-analytic evaluation of longitudinal epidemiological studies. J Affect Disord. 2011; 135: 10-19.
4. Taylor DJ, Lichstein KL, Durrence HH, et al. Epidemiology of insomnia, depression, and anxiety. Sleep. 2005; 28: 1457-1464.
5. Roth T. Insomnia: definition, prevalence, etiology, and consequences. J Clin Sleep Med. 2007; 3: S7-10.
6. Leger D, Bayon V. Societal costs of insomnia. Sleep Med Rev. 2010; 14: 379-389.
7. Riemann D, Voderholzer U, Spiegelhalder K, et al. Chronic insomnia and MRI-measured hippocampal volumes: a pilot study. Sleep. 2007; 30: 955-958.
8. Winkelman JW, Benson KL, Buxton OM, et al. Lack of hippocampal volume differences in primary insomnia and good sleeper controls: an MRI volumetric study at 3 Tesla. Sleep Med. 2010; 11: 576-582. 9. Altena E, Vrenken H, Van Der Werf YD, et al. Reduced orbitofrontal and parietal gray matter in chronic insomnia: a voxel-based morphometric study. Biol Psychiatry. 2010; 67: 182-185.
10. Joo EY, Noh HJ, Kim JS, et al. Brain Gray Matter Deficits in Patients with Chronic Primary Insomnia. Sleep. 2013; 36: 999-1007.
11. Dai XJ, Peng DC, Gong HH, et al. Altered intrinsic regional brain spontaneous activity and subjective sleep quality in patients with chronic primary insomnia: a resting-state fMRI study. Neuropsychiatr Dis Treat. 2014; 10: 2163-2175.
12. Wang T, Li S, Jiang G, et al. Regional homogeneity changes in patients with primary insomnia. Eur Radiol. 2016; 26: 1292-1300.
13. Li C, Ma X, Dong M, et al. Abnormal spontaneous regional brain activity in primary insomnia: a resting-state functional magnetic resonance imaging study. Neuropsychiatr Dis Treat. 2016; 12: 1371-1378.
14. Nofzinger EA, Buysse DJ, Germain A, et al. Functional neuroimaging evidence for hyperarousal in insomnia. Am J Psychiatry. 2004; 161: 2126-2128.
15. Altena E, Van Der Werf YD, Sanz-Arigita EJ, et al. Prefrontal hypoactivation and recovery in insomnia. Sleep. 2008; 31: 1271-1276.
16. Killgore WD, Schwab ZJ, Kipman M, et al. Insomnia-related complaints correlate with functional connectivity between sensory-motor regions. Neuroreport. 2013; 24: 233-240.
17. Chen MC, Chang C, Glover GH, et al. Increased insula coactivation with salience networks in insomnia. Biol Psychol. 2014; 97: 1-8.
18. Wang T, Yan J, Li S, et al. Increased insular connectivity with emotional regions in primary insomnia patients: a resting-state fMRI study. Eur Radiol. 2017; 27: 3703-3709.
19. Fan L, Li H, Zhuo J, et al. The Human Brainnetome Atlas: A New Brain Atlas Based on Connectional Architecture. Cereb Cortex. 2016; 26: 3508-3526.
20. Buysse DJ, Reynolds CF, Monk TH, et al. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989; 28: 193-213.
21. Bastien CH, Vallieres A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001; 2: 297-307.
22. McCall WV. A psychiatric perspective on insomnia. J Clin Psychiatry 62 Suppl. 2001; 10: 27-32.
23. Buysse DJ, Thompson W, Scott J, et al. Daytime symptoms in primary insomnia: a prospective analysis using ecological momentary assessment. Sleep Med. 2007; 8: 198-208.
24. Riemann D, Spiegelhalder K, Feige B, et al. The hyperarousal model of insomnia: a review of the concept and its evidence. Sleep Med Rev. 2010; 14: 19-31.
Figures