2341
Brain functional connectivity signatures of neuropathic pain-induced depression in a preclinical model1ICube, University of Strasbourg, Strasbourg, France, 2Dept. of Radiology, Medical Physics, University Medical Center Freiburg, Freiburg im Breisgau, Germany, 3INCI, University of Strasbourg, Strasbourg, France, 4Faculty of Biology, University of Freiburg, Freiburg im Breisgau, Germany, 5Hautepierre Hospital, Department of Biophysics and Nuclear Medicine, Faculty of Medicine, University Hospital Strasbourg, Strasbourg, France
Synopsis
Chronic pain disorders are associated with high prevalence of depression which points to a link between two pathologies; although the underlying mechanisms remain elusive. As a translational approach, preclinical MR imaging offers a unique opportunity to reliably establish causal relations between the pathological conditions and brain function in vivo. In this study, we used a mouse model of neuropathic pain to investigate affective consequences of chronic pain. We performed behavioural assessments as well as resting-state fMRI and our results show a remodelling of functional connectivity in regions belonging to default-mode network and the reward system in mice with pain-induced depression.
Introduction
Chronic pain is a prevalent determinant of depressive disorder: for instance, around 30% of patients with neuropathic pain (e.g. radiculopathies, diabetic neuropathy) develop depression1. There is a paucity of information regarding the precise mechanisms underlying these comorbidities, particularly at the brain networks level. So far, resting-state functional Magnetic Resonance Imaging (rs-fMRI) remains the only non-invasive approach, able to probe large scale brain functional connectivity patterns2. It allows mapping the architecture of brain functional networks and their remodelling in various pathological states. As a translational approach, cuff model of neuropathic pain gives rise to chronic pain and anxio-depressive phenotype in a time-dependent manner3. Here, we used rs-fMRI to investigate brain functional connectivity of mice with neuropathic pain-induced depression to identify pathology to network signatures. We aim to shed light on potential mechanisms underlying chronic pain and depression comorbidity.Methods
Experimental procedures: Cuff surgery was performed under ketamine/xylazine (80/10 mg/kg) in C57BL/6J adult male mice; a polyethylene cuff was placed around right main branch of sciatic nerve (cuff or the depressed group, n=7). The nerve was simply exposed for the controls (sham group, n=8). Chronic pain was surveyed with von Frey behavioural test: cuff animals displayed reduction for pain thresholds on the operated paws. Depressive-like phenotype was assessed using novelty-supressed feeding test (NSF) eight weeks after the surgery and rs-fMRI immediately followed.
MRI data acquisition: Rs-fMRI was carried out with a 7T Bruker BioSpec 70/30 USR scanner and room temperature surface coil (Bruker, Ettlingen, Germany). Scans were done under medetomidine (0.15 mg/kg sc bolus, 0.3 mg/kg/h sc infusion); respiration and body temperature were monitored throughout. Acquisition parameters were: GE-EPI sequence, 31 axial slices, FOV=2.12×1.8 cm, matrix=147× 59, TE/TR= 15 ms /2000 ms, 500 image volumes, 0.14× 0.23× 0.5 mm³ resolution. Acquisition time was 16 minutes. T2-weighted images and diffusion tensor images were also acquired in the same scanning session.
Data processing: Rs-fMRI images were spatially normalized into a template using ANTs4 and smoothed (FWHM=0.28×0.46×1 mm3) with SPM8. Seed-based functional connectivity (FC) analysis was performed using regions of interest (ROI) extracted from Allen Mouse Brain Atlas which were normalized into the template space. Resting-state time series were de-trended, band-pass filtered (0.01-0.1 Hz) and regressed for cerebrospinal fluid signal from ventricles. Partial correlation between the mean time course of each ROI and each voxel of the brain was computed at the group and individual levels. Individual connectivity maps were used for statistical comparison with SPM8.
Results and Discussion
ROI analysis revealed differential patterns of connectivity for several brain regions. Fig.1 shows connectivity of retrosplenial area (RSP) which is considered the core region for rodent default-mode network (DMN)5. Depressed mice display a modified DMN pattern, with enhanced cross-talk between this RSP and the rostral structures: anterior cingulate area (ACA) and medial prefrontal regions (mPFC). Similar observations on stronger connectivity of prefrontal parts of DMN were documented across several chronic pain conditions6 and major depression7,8 in human studies.
Reward and aversion systems9, relying on the well-known meso-cortico-limbic circuitry have been implicated in both major depression10 and chronic pain11. Here we demonstrate significant remodelling of FC patterns of core players of the reward/aversion system (Fig. 2). Notably, ventral tegmental area (VTA) of depressed animals (Fig. 2A) exhibited significant changes in connectivity towards rostral limbic regions, including nucleus accumbens (NAc) and prefrontal cortex when compared to sham mice. We found that, NAc, the well-known hub of the reward circuitry, modifies its connectivity with ACA, RSP and VTA in the depressed group (Fig. 2B). Lateral habenula (LHb), a newly identified region implicated in aversion circuitry, demonstrated large FC changes with ACA, RSP, striatum, and hippocampus as compared to shams (Fig. 2C). Moreover, LHb - NAc/septal connectivity is significantly impaired in depressed animals.
Conclusions and Perspectives
Our results indicate a remodelling of DMN and reward circuitry in pain-induced depression. Such modifications might explain behavioural phenotype seen in the model3: cuff mice display lack of motivation and anxiety in the NSF test and reduced grooming behaviour-also a surrogate of motivation- in the splash test. Numerous candidate synaptic(e.g. BDNF; cytokine signalling-induced plasticity) and molecular mechanisms(e.g. CREB expression, chromatin modifications) brought forth by research focused on reward circuitry alterations in mood disorders12 could be at the source of circuit-level changes we observed in our study.
Further analysis of diffusion tensor imaging data - acquired in parallel for this mice cohort – is warranted, in order to examine the structural scaffolding and possible structural connectivity changes underlying functional alterations.
Acknowledgements
No acknowledgement found.References
1. Radat, F., Margot-Duclot, A. & Attal, N. Psychiatric co-morbidities in patients with chronic peripheral neuropathic pain: A multicentre cohort study: Psychiatric co-morbidities and neuropathic pain. Eur. J. Pain n/a-n/a (2013).
2. Smith, S. M. et al. Functional connectomics from resting-state fMRI. Trends Cogn. Sci. 17, 666–682 (2013).
3. Yalcin, I. et al. A time-dependent history of mood disorders in a murine model of neuropathic pain. Biol. Psychiatry 70, 946–953 (2011).
4. Avants, B. B. et al. A reproducible evaluation of ANTs similarity metric performance in brain image registration. Neuroimage 54, 2033–2044 (2011).
5. Stafford, J. M. et al. Large-scale topology and the default mode network in the mouse connectome. Proc. Natl. Acad. Sci. 111, 18745–18750 (2014).
6. Baliki, M. N., Mansour, A. R., Baria, A. T. & Apkarian, A. V. Functional reorganization of the default mode network across chronic pain conditions. PloS One 9, e106133 (2014).
7. Zhu, X. et al. Evidence of a Dissociation Pattern in Resting-State Default Mode Network Connectivity in First-Episode, Treatment-Naive Major Depression Patients. Biol. Psychiatry 71, 611–617 (2012).
8. Wang, L., Hermens, D. F., Hickie, I. B. & Lagopoulos, J. A systematic review of resting-state functional-MRI studies in major depression. J. Affect. Disord. 142, 6–12 (2012).
9. Berridge, K. C. & Kringelbach, M. L. Pleasure Systems in the Brain. Neuron 86, 646–664 (2015).
10. Zhang, W.-N., Chang, S.-H., Guo, L.-Y., Zhang, K.-L. & Wang, J. The neural correlates of reward-related processing in major depressive disorder: A meta-analysis of functional magnetic resonance imaging studies. J. Affect. Disord. 151, 531–539 (2013).
11. Borsook, D. et al. Reward deficiency and anti-reward in pain chronification. Neurosci. Biobehav. Rev. 68, 282–297 (2016).
12. Russo, S. J. & Nestler, E. J. The brain reward circuitry in mood disorders. Nat. Rev. Neurosci. 14, 609–625 (2013).
Figures
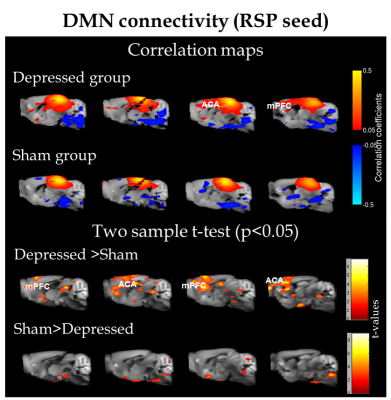
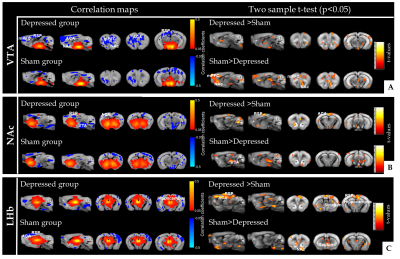
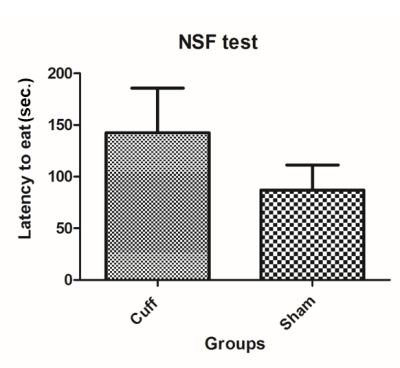
Fig. 3 Novelty suppressed feeding (NSF) test: 24 hours before the behavioural test, food is removed from all the cages. For the experiment, a single pellet of food is laid at the centre of an open field and each mouse is placed in a corner. The latency to touch and eat the pellet is recorded for each animal. This test induces a conflict between the drive to eat the pellet and the fear of venturing to the centre of the field. Anxio-depressive phenotype presents with prolonged latency to eat which was observed in the cuff group in comparison to shams.