2320
Characterization of the Central Analgesic Effects of Two Different Acupuncture Modalities in a Mouse Model of Nociception1Institute of Pharmacology and Toxicology, University of Erlangen-Nuremberg, Bayern - Erlangen, Germany, 2Department of Medicine 3 Rheumatology and Immunology, University Hospital Erlangen, Erlangen, Germany, 3Institute of Molecular Biotechnology, Austrian Academy of Sciences, Vienna, Austria, 4Institute of Acupuncture and Moxibustion, China Academy of Chinese Medical Sciences, Beijing, China, 5Ludwig Boltzmann Institute for Rare and Undiagnosed Diseases, Austrian Academy of Sciences, Vienna, Austria, 6Clinical Institute of Laboratory Medicine, Medical University of Vienna, Vienna, Austria
Synopsis
By using functional MRI, possible analgesic effects of two different acupuncture treatments (insertion of needles and electro-acupuncture) at Zusanli acupoint (ST36) were investigated. The brain’s response in anaesthetized C57Bl/6J mice to noxious stimuli with and without acupuncture was analyzed by characterization of the classical stimulus-driven BOLD parameters but also the influence on stimulus- as well as non-stimulus-driven functional connectivity-based brain networks. Acupuncture was shown to modulate (pseudo-resting state) brain networks by enhancing functional connectivity within limbic structures and decreasing thalamic connectivity particular with electro-acupuncture. Thereby acupuncture exerts control over the processing of noxious stimuli by higher-order brain regions.
Introduction
Acupuncture is based upon the idea of our body's energy flowing in defined paths (meridians). Blockade thereof is believed to evoke several pathologies including chronic pain. Pain being of great burden, the investigation of possible analgesic effects of acupuncture is of great interest. Yet, the underlying mechanisms are barely understood and many studies conducted in humans lack methodological strength and are prone to the placebo-effect. To overcome these flaws, a mouse model was used to investigate the anti-nociceptive effects of acupuncture by fMRI.Materials and Methods
Healthy male C57BL/6J wildtype mice (~3 months), anaesthetized with isoflurane, were measured once via fMRI (4.7T Bruker Biospec, 3cm 2x2 phased array head coil, matrix 64×64, FOV 15×15mm, voxel size 0.234x0.234mm, slice thickness 0.5mm, axial, 22 slices, GE single-shot EPI (TR=200ms; TEef=25.3ms)). During the 50min fMRI session, 12 thermal stimuli of 50°C were applied at the dorsal side of the right hind paw (duration 20sec, inter-stimulus-time 3min 40sec).
Previously the animals were randomly assigned to 4 groups:
1) H (n=3): only the heat stimuli during the measurement.
2) H/N (n=6): one filiform acupuncture needles inserted into the left ST36
acupoint (depth of ~3mm).
3) H/N/EA (n=5): two filiform acupuncture needles inserted, one as described
above and a second ~2mm lateral to the acupoint (similar depth). Both needles
were connected to an electric stimulator generating 1mA 180µs pulses at 2Hz
frequency (electro-acupuncture (EA)) during a 15min time window.
4) N/EA
(n=3): pseudo-resting state (p-RS) measurement. No heat stimuli applied. EA as
described for H/N/EA.
The fMRI time course was split into three time windows
pre-phase: 0-18min
middle-phase: 19-34min (application
of EA in H/N/EA and N/EA group)
post-phase: 35-50min
to check for time-dependencies. For H/N, H/N/EA and N/EA needles were implanted
before the fMRI measurement and retained until end.
Brain responses to the different stimulation conditions were characterized using the same modified Paxinos mouse brain atlas by
I) classical BOLD analysis, comparing the BOLD response amplitude, the activated brain volume of the whole brain and of functionally related sets of brain structures.
II) cross-correlations between average BOLD time courses of significantly activated voxels separately for each of the 196 brain structures resulting in task-driven functional connectivity (FC)-based brain networks [1].
III) multi-seed-region-based pseudo-resting state analysis (MSRA) performed for N/EA group (Kreitz et al. submitted). Changes of FC within networks between groups/phases were assessed at k=10 (i.e. 980 top connections stimulus-driven and 1960 for MSRA).
Results
Initially, the analgesic effect of both acupuncture treatments was quantified by calculating the whole-brain BOLD response amplitude and activated volume for each group and experimental phase. Both acupuncture treatments yielded a significant analgesic effect, which was found to be time-dependent: whereas the H group showed no difference among the three phases, the reduction of both parameters in the two acupuncture groups was greatest in the middle phase respectively (about 50% each). Only H/N/EA showed a persistent reduction in the post-phase, indicating a longer-lasting analgesic effect for EA. Overall, EA was more efficient than needle placement alone.
Task-driven brain networks showed that either method decreased FC between similar brain structures within pre- and middle phase, with the decrease in middle-phase being most prominent (Fig. 3, upper panel). Greatly affected were cortical connections, as well as connectivity to and within basalganglia, dorsal hippocampus and thalamus. This led to changes in information flow within brain networks, represented not by changes in global network parameters (e.g. small-world index, data not shown) but by a shift in hub functionality from prefrontal and limbic areas (H: association cortex, amygdala, hypothalamus, nucleus accumbens) to more cognitive regions (acupuncture: primary somatosensory cortex, hippocampus, cingulate cortex) with enhanced control of thalamus and caudate putamen under EA, thereby influencing directly interactions of brain structures resulting in the anti-nociceptive effect.
P-RS network analysis of N/EA, aimed to identify brain structures directly modulated by EA, revealed interactions of amygdala, hypothalamus, basalganglia and thalamus, hinting at a bottom-up control mechanism of higher-order brain structures originating in limbic structures, acting via thalamus influencing cortical and hippocampal structures. This N/EA effect on the brain at rest might explain the increased analgesic effect of electro-acupuncture even lasting throughout the post-phase.
Conclusion
Analysis revealed a strong but time-dependent analgesic effect of acupuncture at ST36 being stronger for needles plus electro-acupuncture. This analgesia is achieved by controlling higher order brain regions via modulation of a distinct subnet of limbic brain structures.Acknowledgements
BMBF Immunopain (2010-2013; 01EC1004E) and Neuroimpa (2015-2019; 01EC1403C)References
[1] Sporns O, Chialvo DR, Kaiser M, Hilgetag CC. Organization, development and function of complex brain networks. Trends in cognitive sciences 2004;8(9):418-425.Figures
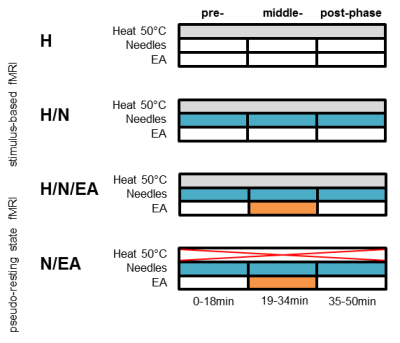
Potential analgesic effects of two different acupuncture modalities (acupuncture needles only (H/N) and electro-acupuncture (H/N/EA)) on noxious thermal heat perception were analyzed by comparing to a third group receiving only the heat stimuli. To account for possible time-dependencies, the experiment was split into three time windows. Pseudo-resting state fMRI (N/EA) was conducted without the heat stimuli but with electro-acupuncture applied during the middle-phase.
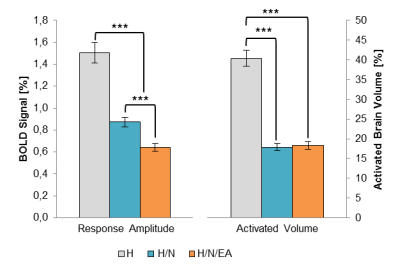
Both acupuncture modalities yielded a highly significant reduction of the classical BOLD parameters response amplitude (left) and activated brain volume (right). Electro-acupuncture was found to be more efficient in further reducing the response amplitude.
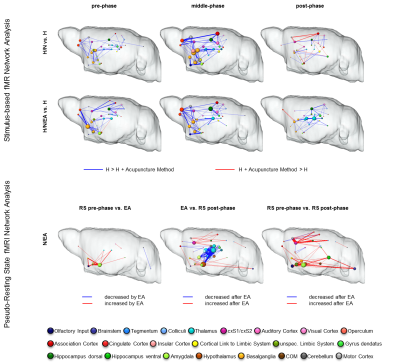
Upper panel: comparison of task-driven brain networks between H group and each acupuncture modality. Functional connectivity (FC) was widely decreased (blue edges) by insertion of needles alone (pre-phase). In both modalities, the analgesic effect was mediated by roughly the same set of brain structures: sensory (pink), motor (grey) and association cortices (red), basalganglia (dark yellow), dorsal hippocampus (dark green) and thalamus (bright blue). However, particular in the post-phase the thalamus was stronger decreased in the H/N/EA group. Lower panel: multi-seed-region-based pseudo-resting state network analysis revealed an EA-mediated significant increase in FC within limbic as well as cortical structures. Thalamic-hippocampal FC was significantly decreased, indicating limbic control over thalamus and thereby also over cortical regions. Enhanced limbic and cortical connectivity lasted after EA, mediating the prolonged analgesic effect of EA.