2315
Functional connectivity and dynamic change of rat brain resting-state networks under morphine-induced condition1Center for Magnetic Resonance Research, Department of Radiology, University of Minnesota, MINNEAPOLIS, MN, United States, 2Department of Biomedical Engineering, Penn State University, State College, PA, United States
Synopsis
Resting-state fMRI (rs-fMRI) in animal is essential for studying neural networks and translational research. However, animal motion poses a major obstacle for performing rs-fMRI, and it is commonly requires anesthesia that could suppress and alter the resting-state networks (RSNs). In this work, we investigated the rat RSNs under morphine condition, and the differentiation and transition of RSNs when animal conditions were changing from isoflurane to morphine. We found that the number of RSNs was significantly increased from deep anesthesia to morphine-induced condition; the RSNs became highly specific to brain functions; and thus, RSN mapping became more reliable.
INTRODUCTION:
Mapping resting-state functional connectivity of animal models is essential for studying comparative functional neuroanatomy and translational research on central nervous system. Despite the success in human resting-state functional magnetic resonance imaging (rs-fMRI) 1,2, functional mapping of animal brains faces many obstacles, in which animal motion during the MRI scanning is a major concern. To restrict animal movement, anesthetics such as isoflurane and medetomidine are normally used; however, the brain networks could be significantly altered or suppressed under anesthetized condition 3,4. To avoid the implication from anesthetics, an alternative approach is to scan well-trained awake animals, which requires extra efforts, time and skills to successfully carry out the study 5-7; nevertheless, head motion is still inevitable in conscious animal. On the other hand, morphine as an opioid-based analgesia agent is able to alleviate the discomfort and stress during the MR scan while having minimum interference on the animal brain networks. In this work, we investigated the resting-state networks (RSNs) generated in rat brains under morphine-induced condition, in particular, the differentiation and transition of the RSNs when animal conditions were changing from isoflurane to morphine.METHODS:
Animals and scan conditions: Two female rats were first scanned under isoflurane anesthesia, and then switch to morphine analgesia. The scan conditions for each rat were changed in following order: 1) 2% isoflurane (Iso20); 2) 1.8% isoflurane (Iso18); 3) 1.5% isoflurane (Iso15); 4) 1.2% isoflurane (Iso12); 5) 1.2% isoflurane with 50 mg/kg bolus injection of morphine (Iso12+Mor50); 6) 0.5% isoflurane with 25 mg/kg/h morphine infusion (Iso05+Mor25); 7) first phase of morphine infusion (Mor25-1); 8) second phase of morphine infusion (Mor25-2); and 9) third phase of morphine infusion (Mor25-3). The physiology of the animal was monitored and well controlled throughout the study.
MRI experiments and data analysis: The MRI experiments were performed on a 9.4T/31cm animal scanner (Varian/VNMRJ) using a single loop (2.5cm diameter) proton surface coil. T2 weighted anatomical images were acquired with TR/TE=3000/12 ms; matrix = 256 × 256; FOV=3.2 × 3.2 cm; 24 slice and 0.75mm thickness. Gradient echo (GE)-echo planar imaging (EPI) based rs-fMRI images were obtained with TR/TE = 1000/17 ms; matrix = 64 × 64; FOV = 3.2 × 3.2 cm; 18 slice and 0.75mm thickness. For each scan condition, one rs-fMRI dataset with 500 volumes was obtained. These rs-fMRI data were preprocessed following the standard pipeline and the RSNs were generated using group independent component analysis (ICA) GIFT 5-7.
RESULTS
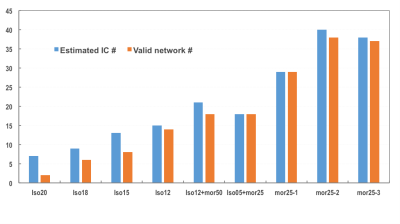
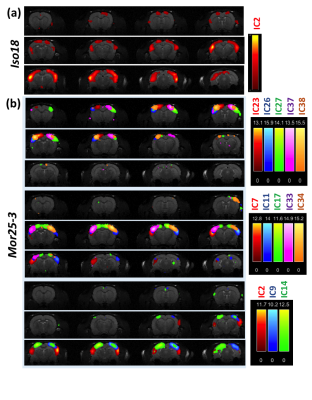
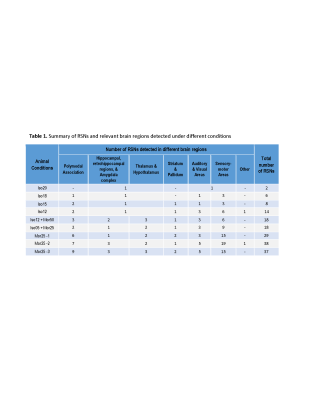
Figure 1 shows the estimated independent components (IC) by principle component analysis (PCA) under each condition and the identified meaningful resting-state networks (i.e. RSNs) that were valid. Clearly, when animal condition changing from deep anesthesia (Iso20) to morphine analgesia (Mor25), the number of RSNs were increasing dramatically. The brain regions involved in these networks and the numbers of RSNs detected under different brain conditions were summarized in Table 1. Figure 2 displays an example of composite maps of RSNs of a representative rat brain under 1.8% isoflurane (2a, Iso18) and 25mg/kg/h morphine infusion (2b, Mor25-3), respectively. It is obvious that the large resting state network with strong connectivity covering multiple sensory cortical regions under Iso18 became well separated into many distinct and more specific RSNs under Mor25-3 condition.DISCUSSION and CONCLUSION
In this study, we found that the head motion became negligible with the rs-fMRI data obtained under the morphine condition. The comparative results and the rs-fMRI maps showing RSNs are reliable and reproducible, and the group analysis was based on two rs-fMRI datasets from two rats collected under the same condition. The high quality of the RSNs mapping is clearly evident in Fig. 2. The number of RSNs observed under the morphine condition is approaching that of RSNs in awake-state 5-7. Currently, we are conducting a comparative study for studying the similarity and difference of RSNs between morphine and awake states. The outcomes will provide the insight into the effect of opioid-based analgesia agents on brain function and their modulation on functional connectivity.Acknowledgements
NIH grants: R01 MH111447 and NS070839, R24 MH106049 and R24 MH106049 S1, P41 EB015894, P30 NS5076408; and the W.M. Keck Foundation.References
1 Biswal, B., Yetkin, F. Z., Haughton, V. M. & Hyde, J. S. Functional Connectivity in the Motor Cortex of Resting Human Brain Using Echo-Planar Mri. Magnet Reson Med 34, 537-541 (1995). 2 Khalili-Mahani, N. et al. Effects of morphine and alcohol on functional brain connectivity during "resting state": a placebo-controlled crossover study in healthy young men. Hum Brain Mapp 33, 1003-1018 (2012).
3 Liu, X., Zhu, X. H., Zhang, Y. & Chen, W. Neural origin of spontaneous hemodynamic fluctuations in rats under burst-suppression anesthesia condition. Cereb Cortex 21, 374-384 (2011). 4 Liu, X., Zhu, X. H., Zhang, Y. & Chen, W. The change of functional connectivity specificity in rats under various anesthesia levels and its neural origin. Brain Topography 26, 363-377, doi:10.1007/s10548-012-0267-5 (2013).
5 Liang, Z., King, J. & Zhang, N. Uncovering intrinsic connectional architecture of functional networks in awake rat brain. J Neurosci 31, 3776-3783 (2011).
6 Liang, Z., Liu, X. & Zhang, N. Dynamic resting state functional connectivity in awake and anesthetized rodents. Neuroimage 104, 89-99 (2015).
7 Ma, Y., Hamilton, C. & Zhang, N. Dynamic Connectivity Patterns in Conscious and Unconscious Brain. Brain Connect 7, 1-12 (2017).
Figures