2312
BOLD-fMRI evaluation of different types of analgesic agents on allodynia-specific pain in a rat chronic pain model1Faculty of Life Sciences, Kumamoto University, Kumamoto, Japan, 2Graduate School of Medicine, The University of Tokyo, Tokyo, Japan
Synopsis
The aims of this study are to reveal the underlying inhibitory mechanism of a compound against the chemokine signal, based on targeted protein structures, and to evaluate the analgesic effect on allodynia-specific responses in a rat chronic pain model, with our BOLD-fMRI-based pain evaluation system using a green laser. An NMR titration analysis demonstrated that the compound strongly binds to the chemokine receptor-binding protein. BOLD-fMRI revealed that the allodynia-specific responses were suppressed by the administration of the compound, in a similar manner to the existing analgesic, pregabalin, with a completely different mechanism of action from that of the compound.
Introduction
Leukocyte chemotaxis is the phenomenon by which leukocytes intrude into and accumulate within inflamed tissue, and it is induced when chemokines bind to their receptors during inflammation. It is an important phenomenon for protection by the immune system. Excessive immune responses targeting the host’s own cells can cause autoimmune diseases, such as rheumatoid arthritis, while suppressive immune responses can cause cancer. In recent years, accumulating studies have revealed the expression, distribution, and function of the chemokines in the spinal cord under chronic pain conditions [1]. Under these conditions, the chemokines produced by astrocytes or neurons activate the chemokine receptors, to enhance the excitatory synaptic transmission of neurons or to activate microglia. Therefore, the chemokine signals are hopeful therapeutic targets for chronic pain. Recently, we obtained the regulatory compound-A, which inhibits the interaction between chemokine receptors and their binding protein and exerts an anti-inflammatory effect. The aim of this study is to reveal the underlying inhibitory mechanism of the compound against the chemokine signal, based on their structures, and to evaluate the analgesic effect on the allodynia-specific response in a rat chronic pain model, with our BOLD-fMRI-based pain evaluation system using a 532 nm green laser [2].Methods
The NMR titration analysis was performed with a 600 MHz spectrometer (Bruker BioSpin). The reserpine-induced myalgia rats with the symptoms of allodynia were converted as reported previously [3]. The rats were ventilated and treated with gallamine and urethane (1.25 g/kg i.p.). Saline was administered (10 mL/kg i.v.), and 30 minutes later the BOLD experiment was performed. MRI experiments were performed with a 7.0 Tesla Bruker BioSpec scanner and a rat brain 4-channel phased array surface coil (Bruker BioSpin). Functional data were acquired with a 4-shot GRE-EPI sequence (TR: 500 ms, TE: 15 ms, FA: 45°, matrix: 64 × 64, FOV: 2.56 × 2.56 cm2, 13 slices, slice thickness: 0.6 mm). The green laser was used to irradiate the left hind paws 5 times for 2 s every 2 minutes, during the scans. After the first BOLD experiment, the rats were treated with pregabalin (10 mg/kg i.v.) or compound-A (20 mg/kg i.p.), and 30 minutes later the same BOLD experiments were performed. The Independent Component Analysis (ICA) was performed with the FSL software. We searched for the brain regions that showed periodic BOLD responses with the frequency of the laser stimulation (8.3 mHz). The BOLD signal intensity was analyzed with the SPM8 software.Results
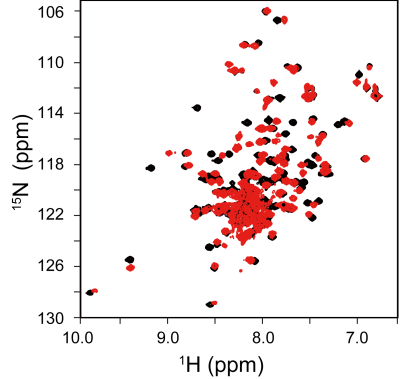
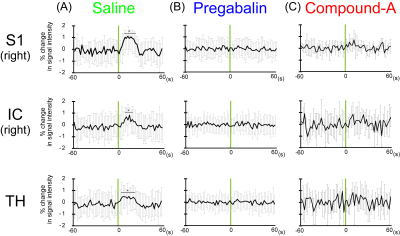
Upon the titration of compound-A to the chemokine receptor-binding protein, many NMR signals from the free protein (Fig. 1, black) were diminished, while the signals from the compound-A-bound protein (Fig. 1, red) appeared in a slow-exchange manner on the NMR time scale. Therefore, compound-A strongly binds to the protein and inhibits its chemokine receptor interaction. Next, we evaluated the analgesic effect of compound-A with our BOLD-fMRI-based pain evaluation system. After the administration of saline, the primary somatosensory cortex (S1), insular cortex (IC), and thalamus (TH) exhibited periodic activation with the frequency of the laser stimulation by ICA. Upon the laser stimulation, the BOLD signal intensities in the S1, IC, and TH were increased by up to about 1% (Fig. 2A). After the rats were treated with an analgesic, pregabalin, no periodic activation was detected by ICA, and no significant BOLD signals were detected in the S1, IC, and TH (Fig. 2B). Similar to the results obtained using pregabalin, compound-A suppressed the laser-evoked S1, IC, and TH signals (Fig. 2C).Discussion
Many more amino acids were affected by the titration than the number expected from the size of compound-A, which indicates that a large conformation change of the chemokine receptor-binding protein is induced by the bound compound. Before the treatments with the agents, the S1, IC, and TH regions were activated by the laser stimulation. S1 is the brain region related to pain sensation, and IC and TH are related to emotion caused by pain. Both pregabalin and compound-A suppressed these pain responses. Pregabalin reportedly inhibits the voltage-dependent calcium channel in neurons, while compound-A inhibits the chemokine signals in neurons or microglia, and thus their mechanisms of action are completely different. Compound-A is expected to have a synergistic effect with existing analgesics.Conclusion
We successfully observed the analgesic effect of the novel chemokine signal-blocking agent on the allodynia-specific responses. Our experimental system provides robust preclinical and clinical evaluations for new analgesic agents.Acknowledgements
No acknowledgement found.References
[1] Zhang Z. et al., Cell. Mol. Life Sci. 74, 3275–3291 (2017)
[2] Yuzuriha N. et al., Proc. Intl. Soc. Mag. Reson. Med. 24, 1722 (2016)
[3] Nagakura Y. et al., PAIN 146, 26–33 (2009)
Figures