2311
The footprint of physiology in ultra-fast RS-FMRI1Radboud University, Nijmegen, Netherlands, 2Donders Centre for Cognitive Neuroimaging, Radboud University, Nijmegen, Netherlands
Synopsis
In the current contribution we study the footprint of physiology in ultra-fast RS-FMRI timeseries by examining RSNs obtained from full brain EPI data acquired with a sampling rate of 158ms.
Purpose
Show the footprint of physiology in RSN timeseries obtained from full brain EPI data acquired with a sampling rate of 158ms.Introduction
The study of functional connectivity with RS-FMRI plays a significant role in cognitive neuroimaging. Nonetheless, exploring the temporal richness of RS data is challenging given the low temporal resolution of FMRI, and our limited knowledge of the interplay between resting state networks, cognitive function, and changes in brain physiology [1]. Detecting, understanding and removing spurious signal confounds - such as motion, respiratory, and cardiac noise - has thus become a popular area of research, and one that benefits the most from accelerated acquisitions capable of critically sampling these phenomena [2].Methods
To critically sample the cardiac signal in humans of all ages we require a sequence with a TR capable of sampling oscillations up to 150bpm, i.e. a TR shorter than 200ms. In addition, to perform full brain functional connectivity analysis we ideally want homogeneous resolution (which excludes inverse imaging [3]), full brain coverage and a TE large enough to capture changes in BOLD contrast. Multiband [4, 5] Echo-Shifted EPI sequence [6] meets all these criteria. Here we propose a protocol with the following parameters: TR = 158ms, TE = 48ms, resolution = 4.5mm^{3}, 30 slices without gap, SMS=6 with a CAIPI FOV/3 shift, no in-plane acceleration and no partial Fourier. The long TE achieved with Echo Shifting allowed us to accelerate even further by deactivating Fat Saturation since the fat signal was already fully relaxed. As a consequence of our short TR we had to lower the flip angle to 8 degrees because of SAR limitations. The long TE and low flip angle thus justify our choice for the spatial resolution of 4.5mm^{3}. With our protocol ready we acquired 54 minutes of resting state data (20480 timepoints in 5 consecutive sessions of 10:30 minutes) from a healthy volunteer using a 32-channel head coil on a 3T Prisma scanner (Siemens, Erlangen, Germany). We also recorded physiological fluctuations using a pulse oximeter and a respiration belt from the scanner manufacturer. Image reconstruction was performed online using the vendor supplied implementation of Slice GRAPPA with Leak Block [7]. A sample image is shown in Figure 1. Our data analysis pipeline consisted of realignment with FSL MCFLIRT, high-pass filtering with a cutoff frequency of 1/100s, and ICA Decomposition using FSL Melodic. We then evaluated the footprint of physiology in ICA components by correlating each component timeseries with physiological recordings and their derivatives, and with these recordings shifted up to 1s in time. For comparison, we also correlated components' timeseries with motion regressors as performed in ICA-AROMA [8].Results
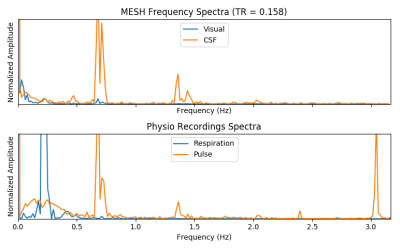
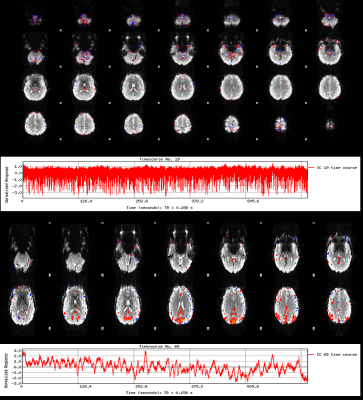
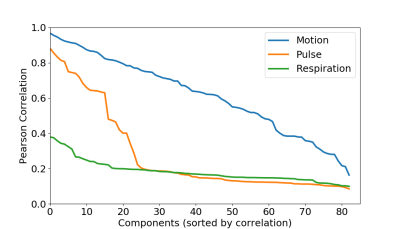
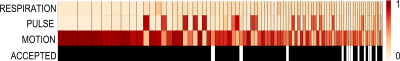
We find IC components whose power spectrum match our physiological recordings, as shown in Figure 2. Spatial maps associated with these components show that the footprint of cardiac pulsations is most notable in regions within or close to ventricles, the base of the brain, and draining veins, as shown in Figure 3. We could reproduce these results in all 5 runs acquired. We found, though, that the footprint of physiology is not as severe as that of motion confounds (Figure 4). Finally, in a approach similar to ICA-AROMA, we show that the physiology footprint can be used to unambiguously classify physiological noise IC components (Figure 5), and that such classification is in good agreement with manual inspection.Discussion
In the current contribution we examined the footprint of physiology in ultra-fast RS-FMRI. We observed that 1. ICA clearly identified the physiological signal, 2. that this signal source does not affect timeseries as much as motion, but can is present in a large number of components, 3.that it is concentrated mainly in the base of the brain and close to draining veins, as also reported in [9]. We also noted that physiological recordings provide extra information that can be used to automatically classify noise components, and that this information is orthogonal to that obtained by motion regressors. We expect to use these methods to explore further benefits of ultra-fast FMRI for the analysis of functional connectivity.Acknowledgements
This study is supported by the Advanced Brain Imaging with MRI (ABRIM) project, funded by EU-Marie Curie Actions and NWO.References
-
[1] Catie Chang and Gary H. Glover. Time-frequency dynamics of resting-state brain connectivity with fMRI. NeuroImage, 50(1):81–98, 2010.
-
[2] Kevin Murphy, Rasmus M. RM Birn, and Peter A. PA Bandettini. Resting-state fMRI confounds and cleanup. NeuroImage, 80:349–359, 2013.
[3] Rasim Boyacioglu and Markus Barth. Generalized iNverse imaging(GIN): Ultrafast fMRI with physiological noise correction. Magn. Reson. Med., 70(4):962–71, oct 2013.
-
[4] David a Feinberg and Kawin Setsompop. Ultra-fast MRI of the human brain with simultaneous multi-slice imaging. Journal of magnetic resonance, 229:90–100, apr 2013.
-
[5] Kawin Setsompop, Borjan A. Gagoski, Jonathan R. Polimeni, Thomas Witzel, Van J. Wedeen, and Lawrence L. Wald. Blipped-Controlled Aliasing in Parallel Imag- ing (blipped-CAIPI) for simultaneous multi-slice EPI with reduced g-factor penalty. Magn. Reson. Med., 67(5):1210–1224, 2012.
-
[6] David G. Norris. Multiband echo-shifted (MESH) EPI. In Proc. Intl. Soc. Mag. Reson. Med. 21 (2013), page 2012, 2013.
-
[7] NickTodd,SteenMoeller,EdwardJ.Auerbach,EssaYacoub,GuillaumeFlandin,and Nikolaus Weiskopf. Evaluation of 2D multiband EPI imaging for high-resolution, whole-brain, task-based fMRI studies at 3T: Sensitivity and slice leakage artifacts. NeuroImage, 124:32–42, 2016.
-
[8] Raimon H.R. Pruim, Maarten Mennes, Daan van Rooij, Alberto Llera, Jan K. Buite- laar, and Christian F. Beckmann. ICA-AROMA: A robust ICA-based strategy for removing motion artifacts from fMRI data. NeuroImage, 112:267–277, 2015.
-
[9] Waqas Majeed, Matthew Magnuson, Wendy Hasenkamp, Hillary Schwarb, Eric H. Schumacher, Lawrence Barsalou, and Shella D. Keilholz. Spatiotemporal dynamics of low frequency BOLD fluctuations in rats and humans. NeuroImage, 54(2):1140– 1150, 2011.
Figures