2307
Arousal-related fMRI modulations contribute to the effect of the motion-based scrubbing on local and long-range connectivity1Biomedical Engineering, Pennsylvania State University, State College, PA, United States
Synopsis
Head motion has been shown to be associated with distinct changes in local and long-range rsfMRI connectivity, and the temporal scrubbing based on motion parameters has been proposed to remove such “motion-induced” artefacts. Here, we showed that scrubbing arousal-related time points resulted in a similar but stronger change on the rsfMRI connectivity than the motion-based scrubbing. Moreover, the effect of the motion-based scrubbing can be completely removed by retaining the part of scrubbed time points related to arousal changes. The findings suggest that arousal modulations may mediate the association between the motion and rsfMRI connectivity.
Introduction
Head motion has been shown to be associated with increased local and decreased long-range brain connectivity measured by resting-state functional magnetic resonance imaging (rsfMRI)1,2. For this reason, scrubbing motion-affected time points based on motion parameters, such as the framewise displacement (FD) and DVARS, has been widely used to remove motion-induced connectivity changes2. The assumption is that the motion causes artifactual changes in fMRI signals and thus their correlations. However, it was shown recently that the same amount of motion did not induce any connectivity changes across two experimental sessions from the same subjects as it did on two sessions from different individuals, suggesting that the association between the motion and rsfMRI connectivity may result from a third factor of neurophysiological relevance3. A possible candidate for this factor is the arousal modulation, which is likely associated with motions and has also been shown to induce global fMRI changes4 that could be potentially detected by motion parameters as motions. In this study, we test this hypothesis by separating arousal-related fMRI changes using a template-matching approach5 and then examining their impacts on local and long-range rsfMRI connectivity as well as commonly-used motion parameters.Methods
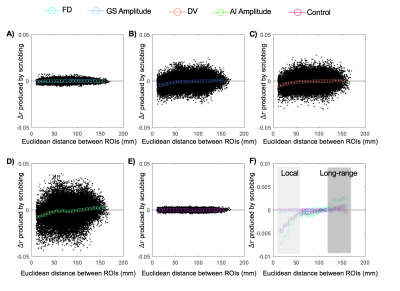
We analyzed the rsfMRI data of the human connectome project (HCP) from 469 subjects who completed all four rsfMRI runs on two sessions. We smoothed the data both spatially (FWHM = 2.4 mm) and temporally (0.001-0.1 Hz) and further standardized each voxel’s signal by subtracting the mean and dividing the standard deviation. Based on the same dataset, we have previously derived a GS co-activation pattern (CAP) that showed very specific deactivations at subcortical wake-promoting regions6. Adapting a template-matching strategy5, we calculated a time course of arousal index (AI) by spatially correlating this GS-CAP with rsfMRI volumes at individual time points. In addition, the FD, DVARS, and GS were also calculated2. Temporal masks were generated to mark 25% time points with highest values in the FD, DVARS, GS amplitude, and AI amplitude respectively. A control mask was also created by circularly shifting the FD mask by 600 time points. Temporal scrubbing was performed with respect to these masks. After the global signal regression, rsfMRI correlations of 264 regions of interests (ROIs) were calculated for scrubbed and unscrubbed data, and the differences (Δr) were summarized as a function of Euclidean distance between ROIs2.Results
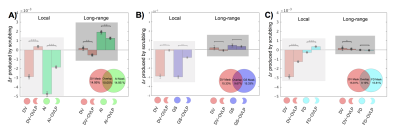
Consistent with previous findings2, temporal scrubbing based on the FD and DVARS reduced the local rsfMRI connectivity but increased the long-range correlations (Fig. 1A and 1C). The scrubbing effect is small for the FD, which is calculated completely based on movement measurements from realignment parameters. In contrast, the DVARS-based scrubbing has a much larger effect, presumably due to the fact that the DVARS is also, per its definition, sensitive to other types of global fMRI changes not caused by the motion. This is consistent with the observation that scrubbing based on the GS amplitude has a similar level of effect as the DVARS-based scrubbing (Fig. 1B). Among all four parameters, the AI-amplitude-based scrubbing showed the largest effect on the rsfMRI connectivity (Fig. 1D), suggesting the key role of arousal-related changes in modulating the local and long-range rsfMRI connectivity. To further investigate interactions between different masks, the temporal scrubbing was also performed in pairs of the masks with excluding the overlapped region. It was found that excluding time points with large DVARS values but retaining those with either big AI amplitudes (Fig. 2A) or large GS amplitudes (Fig. 2B) results in no changes in local and long-range rsfMRI connectivity. Similar result was, however, not observed with excluding the overlap with the FD mask (Fig. 2C).Discussion
The association between the motion and rsfMRI connectivity has been repeatedly observed and assumed to be causal, and the temporal scrubbing based on the motion parameters is now widely applied as a pre-processing step for rsfMRI connectivity analyses7. Here, we tested the hypothesis that the association between the motion and rsfMRI connectivity changes is actually mediated by arousal modulations. Using a GS-CAP template, we located time points showing arousal-related fMRI modulations. Excluding these time points results in reduced local and increased long-range rsfMRI connectivity, similar to but much stronger than removing the same amount of time points associated with high motions. More importantly, the effect of scrubbing high motion time points can be completely removed by selectively retaining about two-fifth of them that are also associated with arousal modulations.Conclusion
The motion-associated rsfMRI connectivity changes can be, at least partly, attributed to brain activity changes associated with arousal modulations.Acknowledgements
This study is supported by the NIH Pathway to Independence Award (K99/R00).References
1. Van Dijk KRA, Sabuncu MR, Buckner RL. The influence of head motion on intrinsic functional connectivity MRI. Neuroimage. 2012;59(1):431-438. doi:10.1016/j.neuroimage.2011.07.044.
2. Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59(3):2142-2154. doi:10.1016/j.neuroimage.2011.10.018.
3. Zeng L-L, Wang D, Fox MD, Sabuncu M, Hu D, Ge M, Buckner RL, Liu H. Neurobiological basis of head motion in brain imaging. Proc Natl Acad Sci U S A. 2014;111(16):6058-6062. doi:10.1073/pnas.1317424111.
4. Liu X, Yanagawa T, Leopold DA, Chang C, Ishida H, Fujii N, Duyn JH. A Spontaneous Neurophysiological Event Underlying Spontaneous fMRI Signal Changes. Proc Organ Hum Brain Mapp 2015 Annu Meet Hawaii, USA. 2015;7439:7439.
5. Chang C, Leopold DA, Scholvinck ML, Mandelkow H, Picchioni D, Liu X, Ye FQ, Turchi JN, Duyn JH. Tracking brain arousal fluctuations with fMRI. Proc Natl Acad Sci U S A. 2016;113(16):4518-4523. doi:10.1073/pnas.1520613113.
6. Liu X, de Zwart JA, Leopold DA, Duyn JH. The global resting-state fMRI signal is associated with opposite changes at subcortical structures regulating arousal. In: In Proceedings of 25th ISMRM Annual Meeting, Honolulu, Hawaii, USA. ; 2017.
7. Power JD, Schlaggar BL, Petersen SE. Recent progress and outstanding issues in motion correction in resting state fMRI. Neuroimage. 2015;105:536-551. doi:10.1016/j.neuroimage.2014.10.044.
Figures