2304
Anesthesia affects connectivity of default-mode sub-networks in the rat in a time-dependent and region-dependent manner1University of Tübingen, Tübingen, Germany, 2Centre d'Imagerie Biomédicale, Ecole Polytechnique Fédérale de Lausanne, Lausanne, Switzerland
Synopsis
Anesthetic agents affect brain connectivity and/or neurovascular coupling, with confounding effects on BOLD resting-state fMRI. To date, the most widespread anesthesia protocol for fMRI in rats consists in isoflurane induction followed by medetomidine sedation. We report that, using this protocol, connectivity of default-mode sub-networks is affected in a time-dependent and region-dependent manner, with modules such as hippocampus becoming detectable as late as two hours into sedation. These spatio-temporal features have significant implications for the interpretation and comparison of resting-state studies in the rat, and of the default-mode network connectivity in particular.
Introduction
The reliable quantification of resting-state (rs) connectivity in the rat brain could open doors to numerous, valuable applications, e.g. measuring functional connectivity changes in rodent models of development, aging, neurodegenerative diseases, stress, etc. One major hurdle is the confounding effect of anesthetic agents on the brain, which have been shown to decrease – but not suppress – functional connectivity strength1,2. To date, the most widespread anesthesia protocol in the rat consists in initial isoflurane induction followed by medetomidine or dexmedetomidine sedation3. Remarkably, a differential effect of isoflurane on types of networks (cognitive and emotional vs sensorimotor) has been reported4, as well as a time-dependent effect of isoflurane and medetomidine/dexmedetomidine on primary somatosensory cortex (S1) connectivity5,6. Here, we study the detectability of local bilateral networks in the rat brain as a function of duration since drug switch from isoflurane to medetomidine.Methods
All experiments were approved by the local Service for Veterinary Affairs and performed on a 14T Varian system using a quadrature surface transceiver. Four adult Sprague-Dawley rats were initially anesthetized with isoflurane (4% induction, 2% maintenance) and set-up in an MRI cradle. After initial adjustments, a bolus of medetomidine (0.1 mg/kg) was injected subcutaneously; isoflurane was discontinued ten minutes later and a continuous infusion of medetomidine (0.1 mg/kg/h) was started 15 minutes after bolus. The total duration of isoflurane delivery was 66 ± 2 min.
For rs-fMRI, two-shot gradient-echo EPI images were acquired as follows: TE=10/12 ms; TR=750 ms; Matrix: 64x64; FOV: 23x23 mm2; 9 slices; slice thickness: 1 mm; 400 repetitions (TA=10’). Multiple runs were acquired on each rat, with time since bolus varying from 33 to 192 min.
Manual brain masking and denoising7 were performed prior to independent component analysis (ICA) in FSL8. Pre-processing steps included removing 10 initial volumes, high-pass filtering (>0.01 Hz), slice timing correction, spatial smoothing (0.7 mm FWHM) and variance normalization of timecourses. Thirty ICs were output, and anatomically relevant ones were selected based on visual inspection.
The percentage of total signal variance (% TV) and Z-score maps for each retained component were analyzed with respect to time since medetomidine bolus.
Long-time between-network connectivity was also estimated by computing the partial correlation matrix of the 30 component timecourses for each run with delay ≥ 150min (4 runs). Partial correlation coefficients (r) were transformed into z-scores, averaged over runs, and transformed back into r.
Results
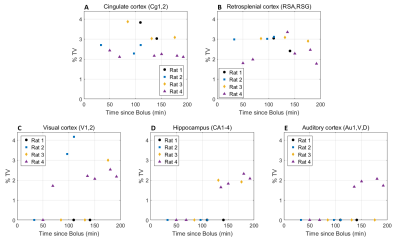
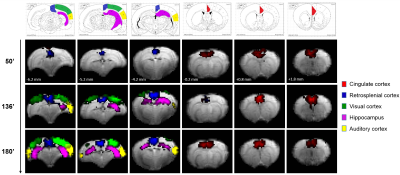
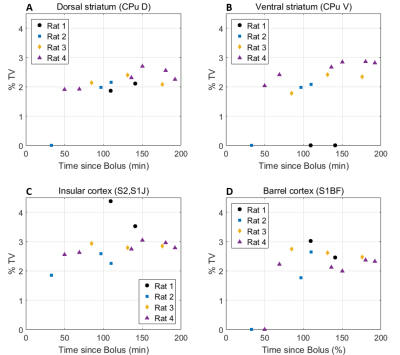
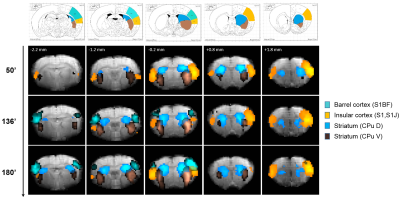
Eleven anatomically-consistent networks were identified, among which were five constituents of the default-mode network (DMN)9-11: cingulate (Cg1,2), retrosplenial (RSA,RSG), visual (V1,2) and auditory (Au1,AuD,AuV) cortices as well as hippocampus (HPC). Other detected networks were striatum – split into ventral and dorsal parts (CPu V, D), insular (S2,S1J), barrel (S1BF), front/hind limb (S1FL,HL) and motor (M1,2) cortices. The separation between the latter two and their bilateralism was variable.
Among the DMN constituents, the cingulate and retrosplenial
cortices were detected from the earliest timepoints, the visual cortex detection
was highly variable between rats (70 min at the earliest), and the auditory cortex and hippocampus were only detected after 120 min
(Figs. 1-2). The other networks were all
detected within one hour of bolus (Figs. 3-4). Increasing the number of IC's did not reveal the late-appearing networks earlier.
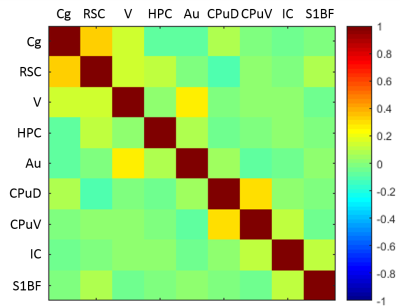
The late-time connectivity matrix revealed significant correlations between cingulate and retrosplenial cortices, and between dorsal and ventral striatum (Fig. 5), as previously reported11.
Discussion and Conclusions
Our results show that local connectivity strength of DMN constituents is affected by anesthetic and/or sedative drugs in a time-dependent and region-dependent manner. A delay of two hours from isoflurane discontinuation and medetomidine infusion was necessary to detect the hippocampus and auditory cortex.
Whether the differences are governed by isoflurane
clearance from the brain (half-time ≈ 20 min12) or gradual
decrease of sedation under constant infusion of medetomidine13 remains
to be established. We note however that the temporal pattern observed
for the DMN does not correlate with α2 receptor
concentration in the brain regions at stake14.
These spatio-temporal features have significant implications for the interpretation and comparison of resting-state studies in the rat, and of DMN connectivity in particular9,11.
Early, intermediate and late-time global connectivity can be compared using a larger cohort of rats. Future work will also focus on the time-dependent connectivity of late-appearing networks using a seed-based approach.
Acknowledgements
The authors thank Analina da Silva, Mario Lepore and Stefan Mitrea for assistance with animal setup and monitoring.This work was supported by the Centre d'Imagerie Bio-Médicale (CIBM) of the University of Lausanne (UNIL), the Swiss Federal Institute of Technology Lausanne (EPFL), the University of Geneva (UniGe), the Centre Hospitalier Universitaire Vaudois (CHUV), the Hôpitaux Universitaires de Genève (HUG) and the Leenaards and the Jeantet Foundations.References
1. Lu H, Zuo Y, Gu H, Waltz JA, Zhan W, Scholl CA, Rea W, Yang Y, Stein EA, 2007. Synchronized delta oscillations correlate with the resting-state functional MRI signal. Proc Natl Acad Sci U S A 104:18265-18269.
2. Liu X, Zhu XH, Zhang Y, Chen W, 2011. Neural origin of spontaneous hemodynamic fluctuations in rats under burst-suppression anesthesia condition. Cereb Cortex 21:374-384.
3. Kalthoff D, Po C, Wiedermann D and Hoehn M, 2013. Reliability and spatial specificity of rat brain sensorimotor functional connectivity networks are superior under sedation compared with general anesthesia. NMR Biomed 26:638-650.
4. Liang Z, Liu X, Zhang N, 2015. Dynamic resting state functional connectivity in awake and anesthetized rodents. Neuroimage 104:89-99.
5. Magnuson ME, Thompson GJ, Pan WJ, Keilholz SD, 2014. Time-dependent effects of isoflurane and dexmedetomidine on functional connectivity, spectral characteristics, and spatial distribution of spontaneous BOLD fluctuations. NMR Biomed 27:291-303.
6. Nasrallah FA, Low SMA, Lew SK, Chen K, Chuang KH, 2014. Pharmacological insight into neurotransmission origins of resting-state functional connectivity: α2-adrenergic agonist vs antagonist. Neuroimage 103:364-373.
7. Veraart J, Novikov DS, Christiaens D, Ades-aron B, Sijbers J, Fieremans E, 2016. Denoising of diffusion MRI using random matrix theory. Neuroimage 142:394-406.
8. Beckmann CF, Smith SM, 2004. Probabilistic independent component analysis for functional magnetic resonance imaging. IEEE Trans Med Imaging, 23(2):137-152.
9. Lu, H., Zou, Q., Gu, H., Raichle, M.E., Stein, E.A., Yang, Y., 2012. Rat brains also have a default mode network. PNAS 109, 3979-3984.
10. Liang X, Hsu LM, Lu H, Sumiyoshi A, He Y, Yang Y, 2017. The rich-club organization in rat functional brain network to balance between communication cost and efficiency. Cereb Cortex.
11. Hsu LM, Liang X, Gu H, Brynildsen JK, Stark JA, Ash JA, Lin CP, Lu H, Rapp PR, Stein EA, Yang Y, 2016. Constituents and functional implications of the rat default mode network. PNAS 113 (31), E4541-E4547.
12. Isoflurane [MAK Value Documentation, 1996]. The MAK-Collection for Occupational Health and Safety. Wiley-VCH Verlag GmbH & Co. KGaA.
13. Pawela, C.P., Biswal, B.B., Hudetz, A.G., Schulte, M.L., Li, R., Jones, S.R., Cho, Y.R., Matloub, H.S., Hyde, J.S., 2009. A protocol for use of medetomidine anesthesia in rats for extended studies using task-induced BOLD contrast and resting-state functional connectivity. Neuroimage 46, 1137-1147.
14. Happe, H.K., Coulter, C.L., Gerety, M.E., Sanders, J.D., O'Rourke, M., Bylund, D.B., Murrin, L.C., 2004. Alpha-2 adrenergic receptor development in rat CNS: an autoradiographic study. Neuroscience 123, 167-178.
Figures