2303
Brain activity and connectivity changes in response to glucose ingestion1Department of Radiology, Leiden University Medical Center, Leiden, Netherlands, 2Department of Methodology and Statistics, Institute of Psychology, Leiden, Netherlands, 3Leiden Institute for Brain and Cognition (LIBC), Leiden, Netherlands, 4Unilever Research & Development, Vlaardingen, Netherlands, 5Department of Internal Medicine, Leiden University Medical Center, Leiden, Netherlands
Synopsis
Understanding of functional brain responses yields insights into satiety signaling, nutrient sensing, energy seeking and feeding behavior. The current aim was to determine normal whole brain functional responses to the ingestion of glucose in healthy normal weight subjects using BOLD signal, network connectivity and Eigen vector centrality functional MRI analysis approaches. Our results show that ingestion of glucose in a fasted state leads to deactivation and decreased connectivity, which can be associated with satiation and reward effects in the brain and a decrease in energy seeking. In contrast, drinking plain water leads to activation and increased centrality and connectivity.
Introduction
The regulatory role of the brain in directing glucose homeostasis, energy homeostasis, and thus eating behavior, is increasingly being recognized.(1) Glucose is the primary source of energy for the brain, and its metabolism is kept under tight regulation. However, energy consumption is not only regulated by homeostatic processes, it also has hedonic aspects in which the brain has an important role.(2-4) Although many areas in the brain have been found to respond to food cues, very little data is available about functional brain responses after actual consumption of energy. It is to be expected that various brain areas that are involved in homeostasis, reward, motivation or inhibition and decision making, show functional responses after glucose ingestion. Understanding of these functional brain responses yields insights into satiety signaling, nutrient sensing, energy seeking and feeding behavior. Moreover, it may aid in the development of neurophysiological markers for (dys-)regulation of these systems in obesity and diabetes type 2. The aim of this study was thus to determine normal whole brain functional responses to the ingestion of glucose in healthy normal weight subjects using several functional MRI analysis approaches.Methods
Twenty-five healthy, normal weight, adult males underwent functional MRI on two separate visits. In a single-blind randomized study setup, participants received either a glucose solution (50gr/300ml of water) or plain water (300ml) at each of two visits after an overnight fast. At each visit resting state functional MRI was performed 10 minutes before and 16 minutes after ingestion of the study interventions. We studied changes in Blood Oxygen Level Dependent (BOLD) signal,(5) changes in voxel based connectivity by Eigenvector Centrality Mapping (ECM),(6) both using a voxel-wise comparison approach, and changes in functional network connectivity (7) by comparing average Z-scores per functional network.Results
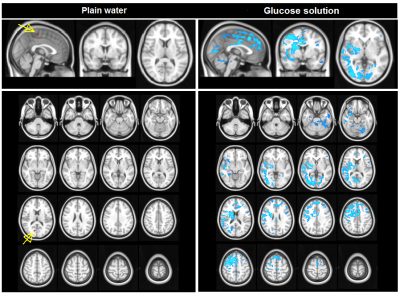
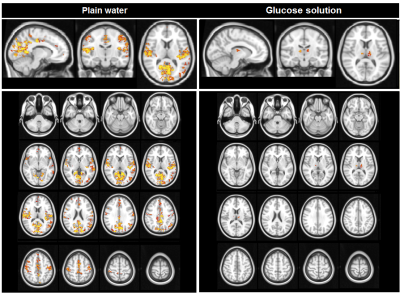
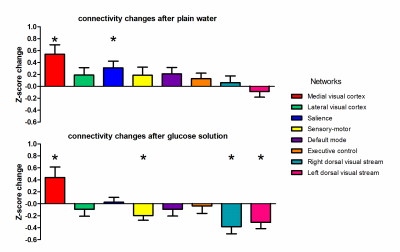
Ingestion of plain water did not lead to BOLD changes. In contrast, ingestion of the glucose solution led to decreases in BOLD signal in clusters containing the insula, thalamus, anterior cingulate gyrus, orbito-frontal cortex, amygdala, hippocampus, and occipital cortex (figure 1). Ingestion of plain water resulted in widespread increases in centrality across the brain, especially in the insula and posterior cingulate cortex, indicating a higher level and quality of voxel-wise connectivity in these areas. Ingestion of glucose solution resulted in a small increase in centrality in the thalamus (figure 2). Analysis of network connectivity showed that ingestion of plain water led to increases in connectivity in the medial and lateral visual cortex network, glucose ingestion led to decreases in connectivity in the sensory-motor and dorsal visual stream networks (figure 3).Discussion
Our data show that the ingestion of both water and glucose led to changes in BOLD activity and functional connectivity on both a voxel-wise and network level throughout the brain. Our results show that after an overnight fast, drinking plain water leads to activation and increased centrality of several brain areas, as well as concerted increased connectivity in brain networks that are associated with energy and reward seeking and expectation. In contrast, ingestion of glucose in a fasted state leads to deactivation and decreased connectivity, which can be associated with satiation and reward effects in the brain and a decrease in energy seeking. Interestingly, the brain areas and networks that show functional responses to consumption of water and glucose in our study have also been implied to function differently in obesity (8-15) and type 2 diabetes.(16;17) This suggest that maintaining the functional responses found in our study could important for maintaining energy and glucose homeostasis.Acknowledgements
The collection of the datasets used in this study was funded by Unilever Research and Development Vlaardingen B.V. The Netherlands. M. Hoeksma and C. Blonk are both employees of Unilever Research and Development Vlaardingen B.V. The Netherlands. All other authors have no conflict of interest to disclose.References
(1) Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW. Central nervous system control of food intake and body weight. Nature 2006;443:289-295.
(2) Avena NM, Rada P, Hoebel BG. Evidence for sugar addiction: behavioral and neurochemical effects of intermittent, excessive sugar intake. Neurosci Biobehav Rev 2008;32:20-39.
(3) Colantuoni C, Rada P, McCarthy J et al. Evidence that intermittent, excessive sugar intake causes endogenous opioid dependence. Obes Res 2002;10:478-488.
(4) Rada P, Avena NM, Hoebel BG. Daily bingeing on sugar repeatedly releases dopamine in the accumbens shell. Neuroscience 2005;134:737-744.
(5) Rombouts SA, Scheltens P, Kuijer JP, Barkhof F. Whole brain analysis of T2* weighted baseline FMRI signal in dementia. Hum Brain Mapp 2007;28:1313-1317.
(6) Wink AM, de Munck JC, van der Werf YD, van den Heuvel OA, Barkhof F. Fast eigenvector centrality mapping of voxel-wise connectivity in functional magnetic resonance imaging: implementation, validation, and interpretation. Brain Connect 2012;2:265-274.
(7) Beckmann CF, DeLuca M, Devlin JT, Smith SM. Investigations into resting-state connectivity using independent component analysis. Philos Trans R Soc Lond B Biol Sci 2005;360:1001-1013.
(8) Garcia-Garcia I, Jurado MA, Garolera M et al. Functional network centrality in obesity: A resting-state and task fMRI study. Psychiatry Res 2015;233:331-338.
(9) Kullmann S, Heni M, Veit R et al. The obese brain: association of body mass index and insulin sensitivity with resting state network functional connectivity. Hum Brain Mapp 2012;33:1052-1061.
(10) Lips MA, Wijngaarden MA, van der Grond J et al. Resting-state functional connectivity of brain regions involved in cognitive control, motivation, and reward is enhanced in obese females. Am J Clin Nutr 2014;100:524-531.
(11) Marques-Iturria I, Scholtens LH, Garolera M et al. Affected connectivity organization of the reward system structure in obesity. Neuroimage 2015;111:100-106.
(12) Stoeckel LE, Weller RE, Cook EW, III, Twieg DB, Knowlton RC, Cox JE. Widespread reward-system activation in obese women in response to pictures of high-calorie foods. Neuroimage 2008;41:636-647.
(13) Wijngaarden MA, Veer IM, Rombouts SA et al. Obesity is marked by distinct functional connectivity in brain networks involved in food reward and salience. Behav Brain Res 2015;287:127-134.
(14) Garcia-Garcia I, Jurado MA, Garolera M et al. Alterations of the salience network in obesity: a resting-state fMRI study. Hum Brain Mapp 2013;34:2786-2797.
(15) Kullmann S, Pape AA, Heni M et al. Functional network connectivity underlying food processing: disturbed salience and visual processing in overweight and obese adults. Cereb Cortex 2013;23:1247-1256.
(16) Cui Y, Li SF, Gu H et al. Disrupted Brain Connectivity Patterns in Patients with Type 2 Diabetes. AJNR Am J Neuroradiol 2016.
(17) Chen Y, Liu Z, Zhang J et al. Selectively Disrupted Functional Connectivity Networks in Type 2 Diabetes Mellitus. Front Aging Neurosci 2015;7:233.
Figures