2301
Human whole-brain sub-millimeter cerebral blood flow map using 7T ASL1Department of Cognitive Neuroscience, Maastricht University, Maastricht, Netherlands, 2SFIM, NIMH, Bethesda, MD, United States
Synopsis
Arterial spin labeling (ASL) offers non-invasive cerebral blood flow (CBF) measurements, but typically suffers from low signal-to-noise ratio, limiting the achievable spatial resolution. In this work, we employ 3D EPI ASL at 7T, partially-overlapping acquisition of multiple slabs and across-session averaging to achieve a high-quality whole-brain 0.7 mm3 isotropic resolution CBF map from a healthy volunteer. The dataset presents the highest spatial resolution CBF map in humans so far, and a unique opportunity to investigate the cortical distribution of baseline CBF across and within brain areas, including providing a physiological basis for the interpretation of laminar and columnar fMRI.
Introduction
Arterial spin labeling (ASL) offers non-invasive cerebral blood flow (CBF) measurements in health and disease1. ASL has notoriously low signal-to-noise ratio (SNR), which limits the achievable spatial resolution or demands long acquisition times. Therefore, obtaining whole-brain sub-millimeter resolution human CBF maps has been considered unfeasible. Strategies to improve ASL’s temporal SNR (tSNR) include 3D readouts2 and ultra-high magnetic fields3. In this study, we achieved, for the first time, the acquisition of sub-millimeter isotropic (0.7 mm3) ASL in humans over the whole brain. Sub-millimeter baseline and activation ASL (f)MRI can be utilized to investigate layer specificity4 and cortical parcellation, as brain areas are known to differ in their vascular density and, hence, in perfusion5.Methods
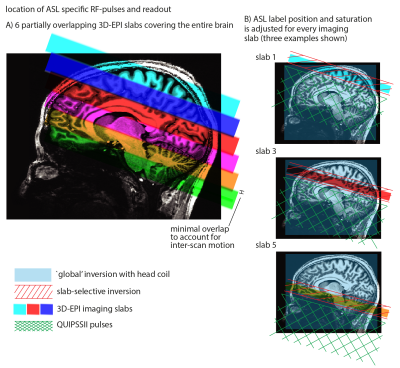
Measurements were performed on a whole-body 7 Tesla MRI scanner (Siemens Healthineers) with a 32-channel receive head-coil (Nova Medical). A 20-year-old female volunteer was scanned in twelve separate sessions after giving informed consent. Each session lasted 2 hours and contained at least six 10-minute FAIR QUIPSS II ASL runs positioned at different locations over the brain to achieve most efficient coverage of the whole brain (Figure 1). One or more runs were repeated in each session to improve the resulting data quality.
Slab-selective (45 mm) or non-selective inversion was accomplished at 7T using an optimized 10 ms tr-FOCI pulse6. In order to further increase the labeling efficiency at 7T, two rectangular 18x18 cm2 high-permittivity 'dielectric pads' with 5 mm thickness were placed on both sides of the head at the level of the temporal lobes7. The acquisition parameters were: 0.7 mm3 isotropic resolution, 36 slices, 19 degrees nominal flip angle, FLASH-GRAPPA 4, TE/TI1/TI2/TR = 16/700/1890/3000 ms, 61 ms scan-time per slice, 200 TRs. M0 images were also acquired with identical acquisition parameters apart from: inversion and saturation pulses switched off, 192 slices, TR = 12.6 s and 20 TRs. All data within a session were motion-corrected and coregistered with SPM 8 using 6 degrees of freedom (dof) to the M0. Perfusion-weighted data were normalized with its respective M0 before averaging across sessions. Data across sessions were coregistered to the mean M0 (Figure 2) with ANTs using 9 dof8 to account for differences in distortions. Voxel-wise CBF, control-image and perfusion tSNR maps were generated after averaging across sessions.
Results
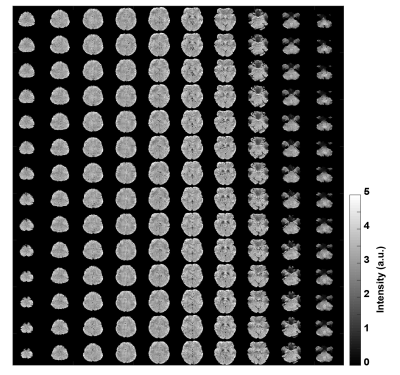
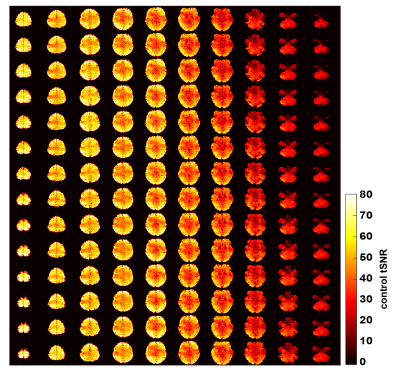
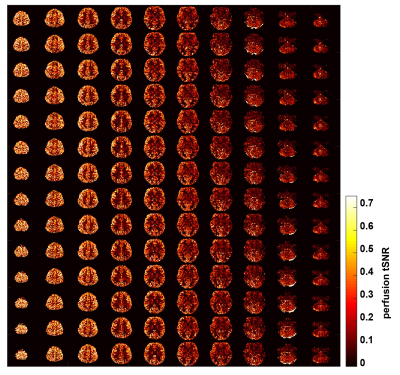
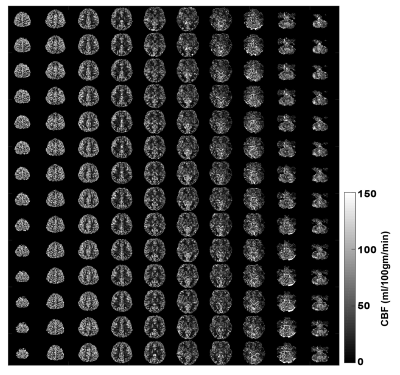
Figure 3 depicts the control tSNR map after averaging across all sessions. The low control tSNR in the basal ganglia is due to short T2* in these regions, while in the inferior temporal lobes it is due to the low transmit B1 there. Figure 4 presents the mean perfusion tSNR map, which generally follows the mean control tSNR, indicating high labeling efficiency across the entire brain. Figure 5 shows the mean baseline CBF in ml/100g/min with clear delineation of the grey matter ribbon. Due to minimal partial volume effects with white matter and CSF, grey matter perfusion reaches values above 100 ml/100g/min. Nevertheless, values above 200 ml/100g/min are primarily seen in venous vessels.Discussion
This work presents the first whole-brain sub-millimeter human CBF map. It was obtained at 7T by concatenating several partially-overlapping 3D EPI ASL slabs and averaging across multiple sessions. The 3D EPI readout offers higher tSNR, due to the volumetric excitation and reduced slice-dependent perfusion-signal loss and coverage compared to 2D EPI9. The 3D readout also maximizes the temporal efficiency by lasting throughout almost the entire available imaging time. High in-plane resolution necessitates using GRAPPA 4 to shorten the EPI echo-train and reduce the TE, distortions, blurring and the scan-time per slice. In case a limited portion of the brain is of interest, even one two-hour session may suffice. The CBF data quality varies across the brain partly due to the different number of averages contributing, but mostly due to remaining B1+-inhomogeneities. Low B1+ prevents not only adequate spin labeling, but also leads to poor imaging data at 7T in areas, such as the inferior temporal lobes. Acquiring multiple small slabs across the brain decouples these two effects, offering improved labeling efficiency for the ones positioned superior to the thalamus. Further improvement of the labeling and imaging quality may be offered by parallel transmission techniques10.Conclusion
High-quality large-coverage sub-millimeter isotropic CBF mapping in humans can be efficiently achieved by 3D EPI ASL at 7T. The approach will help determine the utility of CBF for cortical parcellation and the unprecedented 0.7 mm3 resolution will provide invaluable knowledge about the mesoscopic brain perfusion, which is vital for the interpretation of laminar and columnar fMRI.Acknowledgements
This research has been financially supported by VIDI grant (452-11-002) to KU. We thank Andrew Webb for providing us with the dielectric pads used in this study. We acknowledge help by Markus Barth and Josef Pfeuffer on earlier versions of the 3D EPI ASL sequence code used here.References
1. Alsop et al., MRM, 2015, 73:102-16. Recommended Implementation of Arterial Spin Labeled Perfusion MRI for Clinical Applications: A consensus of the ISMRM Perfusion Study Group and the European Consortium for ASL in Dementia.
2. Vidorreta et al., Neuroimage, 2013, 66:662-71. Comparison of 2D and 3D single-shot ASL perfusion fMRI sequences.
3. Ivanov et al., Neuroimage, 2017, 156:363-376. Comparison of 3T and 7T ASL techniques for concurrent functional perfusion and BOLD studies.
4. Huber et al. Neuroimage, 2017, in press, doi: 10.1016/j.neuroimage.2017.07.041. Non-BOLD contrast for laminar fMRI in humans: CBF, CBV, and CMRO2.
5. Gusnard and Raichle, Nat Rev Neurosci. 2001, 2:685-94. Searching for a baseline: functional imaging and the resting human brain.
6. Hurley et al., MRM, 2010, 63:51-8. Tailored RF pulse for magnetization inversion at ultrahigh field.
7. Teeuwisse et al., MRM, 2012, 67:912-8. Simulations of High Permittivity Materials for 7T Neuroimaging and Evaluation of a New Barium Titanate-Based Dielectric.
8. Avants et al., Neuroimage, 2011, 54:2033-44. A reproducible evaluation of ANTs similarity metric performance in brain image registration.
9. Ivanov et al., Proceedings of the High Field Meeting of the ISMRM, 2016, p. 14. Sub-millimeter human brain perfusion imaging using arterial spin labelling at 3 and 7 Tesla.
10. Tse et al., MAGMA, 2016, 29:333-45. Volumetric imaging with homogenised excitation and static field at 9.4 T.
Figures