2290
Quadrupolar relaxation enhancement in selected Bismuth-Aryl compounds: Promising precursors for novel T1 MRI contrast agents1Institute of Medical Engineering, Graz University of Technology, Graz, Austria, 2Faculty of Mathematics and Computer Science, Universtiy of Warmia and Mazury, Olsztyn, Poland, 3Institute for Chemistry and Technology of Materials, Graz University of Technology, Graz, Austria, 4Institute of Paper, Pulp and Fibre Technology, Graz University of Technology, Graz, Austria
Synopsis
209Bi-aryl compounds have the potential for designing novel class of smart MRI T1 contrast agents which are sensitive to the chemical environment and the B0 field. We have confirmed quadrupolar relaxation enhancement (QRE) of protons as the underlying mechanism in two solid organobismuth-compounds in the B0 range 0.5 – 3T. We also show first QRE peaks of solvent protons in a solution of Tris-(2-orthomethoxy-Phenyl)Bismuthane in tetrahydrofurane. This very important first step yields two promising candidates for the development of QRE-based CAs and opens the way for the second step, i.e. grafting them onto water-soluble nanoparticles for optimizing the relaxivity.
INTRODUCTION
High spin quadrupolar nuclei (QN) such as 209Bi have the potential to provide quadrupolar T1 relaxation enhancement (QRE) of protons and can serve as cores of a entirely novel class of MRI T1 contrast agents (CA)1. Condition for QRE is a high probability for quantum-mechanical transitions of the 209Bi at the 1H Larmor frequency fL or its second harmonic 2fL. In this case the magnetization transferred from 1H to 209Bi by fluctuating dipole-dipole coupling (DDC) is efficiently relaxed by the QN’s quadrupolar relaxation2. The effect depends on flux density B0 , proton exchange rate, the motional dynamics of the molecule and the electric field gradient (EFG) caused by the chemical bonding structure of the QN. Therefore QRE can enable smart CAs for molecular MRI by switching on and off the contrast either by B0 shifts or by chemical interaction with the biological environment. As important steps towards a QRE based CA we (1) pre-selected two promising organo-Bismuth-compounds and determined their quadrupole transition frequencies QTF by Nuclear Quadrupole Resonance Spectroscopy (NQRS), (2) identified QRE in solid powders by Fast Field Cycling NMR spectroscopy (FFC-NMRS) and (3) observed for the first time QRE in solution. The solid QRE spectra were interpreted with a quantum-mechanical model for the expected transition probabilities.METHODS
Tris-(2-orthomethoxy-Phenyl)Bismuthane and Tris-(2,6-Diorthomethoxy-Phenyl)Bismuthane were synthetized and checked for purity by NMR spectroscopy. Their quadrupolar coupling constant Qcc and asymmetry parameter η were determined from their QTF by NQRS with a Tecmag Scout spectrometer and custom-built coils3. FFC-NMRS was carried out on a STELAR Spinmaster relaxometer equipped with a magnet operating up to 3T, at frequencies 20MHz – 128MHz (ca. 0.5 – 3T). For comparison with the FFC-NMRS data a simple quantum-mechanical model was employed: Because of the absence of rotations in the solid the energy level structure of the Bi was assumed to be static. The spectral density of DDC by the remaining modes of molecular motion was considered as frequency-independent. Thus we calculated the quantum-mechanical transition probabilities TP(B0) between energy eigenstates of the 209Bi at fL and 2fL (corresponding to single and double 1H quantum transitions) from Qcc and η. The angle-depencence of QRE was considered by integrating over a spherically uniform random distribution of the EFG orientation, as expected in powders. Results were presented in normalized form TPnorm=TP(B0)/max(TP).RESULTS
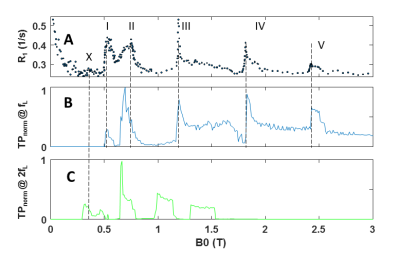
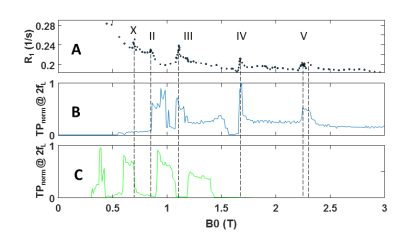
Fig. 1A shows peaks of R1 labelled I – V in the measured R1(B0) of (Tris-(2-orthomethoxy-Phenyl)Bismuthane. The simulated TPnorm for 209Bi transitions at fL in fig. 1B shows a very similar pattern with the same peak locations except for a slight downshift of peak II. TPnorm at 2fL in fig. 1C is expected to contribute much more weakly to R1 because of the much lower probability for 1H double quantum transitions. However, the weak features labelled with ‘X’ may be due to such transitions. The steep increase of the relaxation rate below 0.2T is due to dipolar proton-proton interaction which is known to cause strong background relaxation at low B0. Fig. 2 shows the same information for Tris-(2,6-diorthomethoxy-Phenyl)Bismuthane. The measured peaks III- IV again occur at the frequencies of high TPnorm for single fL. Peak II appears upshifted in the simulation and feature ‘Y’ coincides only with the edge of a peak in fig. 2.C, i.e. a 1H double quantum transition.Discussion
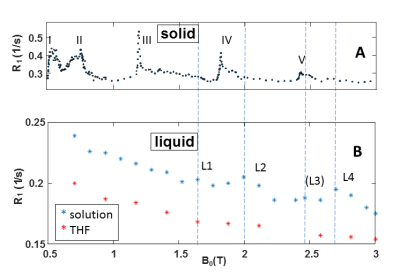
Despite strong model simplifications the agreement between simulated and experimentally observed peaks is very good and confirms QRE of intrinsic protons in two solid organo-Bismuth compounds. We thus consider them as promising candidates for showing QRE after grafting them onto nanoparticles which can be dispersed in water. Preliminary FFC-NMRS data of a solution of (Tris-(2-orthomethoxy-Phenyl)Bismuthane in tetrahydrofurane (THF) at -73°C in fig. 3B show two clear broad peaks L2 and L4 and two smaller ones (L1, L3) at B0 > 1.5T. Their locations are close to but not identical with those of peaks IV and V of the solid (fig 3A), which is to be expected due to fast rotation of the particles in solution.CONCLUSION
To our knowledge we have successfully demonstrated, for the first time, QRE in two selected solid organobismuth compounds. Moreover we found QRE in a solution of (Tris-(2-orthomethoxy-Phenyl)Bismuthane in THF. Remarkeably the last peak occurs at 2.7T, i.e. close to the clinical 3T. Though the effect in the liquid is still small due to strongly suboptimal particle dynamics, this is an important step towards the development of QRE based T1 CAs. The next steps are the preparation of suitable nanoparticles for the maximization of the relaxivity and tuning to concrete clinical field strengths.Acknowledgements
This project has received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement No 665172.References
[1] C. Gösweiner, D. Kruk, P. Westlund, R. Fischer, M. Schlögl, M. Bödenler, A. Petrovic, S. Spirk, H. Scharfetter. ‘Extrinsic MRI Contrast Agents Based on Nuclear Quadrupole Enhanced Relaxation: Principle, Requirements and Characterization of Promising Compounds’, Proc. Intl. Soc. Mag. Reson. Med. 25, 3058, 2017.
[2] D. Kruk, A. Kubica, W. Masierak, A. F. Privalov, M. Wojciechowski, and W. Medycki, ‘Quadrupole relaxation enhancement—application to molecular crystals’, Solid State Nucl. Magn. Reson., 40, 114–120, 2011.
[3] H. Scharfetter, ‘An electronically tuned wideband probehead for NQR spectroscopy in the VHF range’, J. Magn. Reson. 271, 90–98, 2016.
Figures