2285
Wideband mechanical tests of the viscoelastic powerlaw behavior of phantom materials for Magnetic resonance elastography1Department of Radiology, Charité - Universitätsmedizin Berlin, Berlin, Germany, 2Institute for Medicine and Engineering, University of Pennsylvania, Philadelphia, PA, United States, 3Institute of Medical Informatics, Charité - Universitätsmedizin Berlin, Berlin, Germany
Synopsis
Shear rheometry was combined with magnetic resonance elastography (MRE) in a 1.5-T clinical system and a 0.5-T tabletop MRE system to investigate the viscoelastic powerlaw behavior of heparin and polyacrylamide (PAAm) over more than three orders of magnitude dynamic range. While heparin has softer properties than encountered in soft in-vivo tissues, crosslinked PAAm has similar stiffness as measured for in-vivo tissues, however, with lower dispersive properties. Overall both materials are good candidates for the use as standard phantom materials in MRE due to their well predictable springpot properties across the full frequency range relevant for MRE investigations.
Introduction
The viscoelastic springpot model is a powerlaw which describes the mechanical properties of many biological tissues across a wide range of frequencies. The model provides two independent parameters, the shear modulus μ (Pa) and a powerlaw exponent α (dimensionless), which are related to the stiffness and dispersion of biological soft tissue, respectively. Springpot-based viscoelastic constants have been reported in-vivo for human liver with μ = 4.63±0.91kPa and α = 0.27±0.01 and human brain with μ = 5.58±0.90kPa and α = 0.29±0.01.1 This study is motivated by the need for phantom materials in MRE which reproduce the viscoelastic powerlaw behavior of soft tissue in a wide dynamic range. We investigated heparin gel and polyacrylamide as viscoelastic phantom-materials, analyze their powerlaw behavior over a wide frequency range and compare our findings with a commercially available phantom.Methods
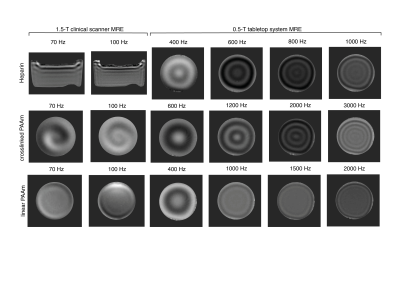
We investigated heparin gel (180000 i.e./100g heparin-sodium, Ratiopharm, Ulm, Germany) and two polymer network samples made of polyacrylamides (PAAm): (i) linear PAAm, i.e. purely linear polymerized PAAm (10 weight percent) and, (ii) crosslinked PAAm, i.e. a composite gel, prepared from crosslinked PAAm polymerized in a solution of linear polymerized PAAm (5%) and water (15, 64, and 14 weight percent, respectively). In addition, a commercially available phantom, CIRS Model-049 phantom (CIRS, Norfolk, Virginia, USA) was investigated for its matrix stiffness, neglecting inclusions. To obtain data in a wide dynamic range we investigated heparin and both PAAm samples by combining experiments from three modalities: (i) oscillatory shear rheometer (MCR 301, Anton Paar, Austria) for 0.08 to 80Hz, (ii) 1.5-T-MRE (Siemens Sonata, Siemens Erlangen, Germany) for 50 to 200Hz and, (iii) 0.5-T compact tabletop MRE,2, for 200 up to 3000Hz dynamic range. Overall a wide frequency range of more than 11 octaves and three orders of magnitude was covered. For both MRE systems, harmonic vibrations were excited by an external piezoelectric driver and captured by motion sensitive phase-contrast MRI (see figure 1). MRE postprocessing was based on analytical fits of shear waves in order to avoid discretization artifacts and noise biases frequently encountered in direct-inversion MRE.3 The complex shear modulus G* was obtained as a function of frequency which was fitted by the springpot model.Results
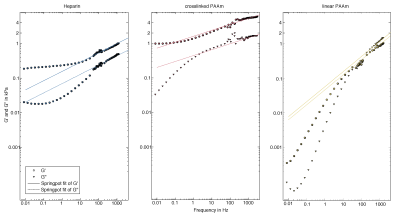
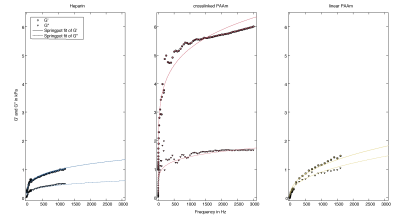
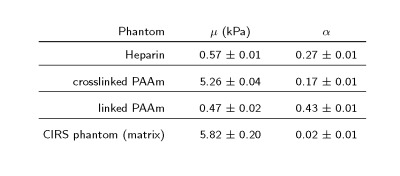
Figures 2,3 show the measured shear modulus dispersion functions for heparin gel and both PAAm polymer network samples including springpot fits demonstrating the wide range of powerlaw behavior of heparin and crosslinked PAAm. Deviations from the powerlaw at very low frequencies (< 1 Hz) are expected since the springpot does not support static stress. In contrast to heparin and crosslinked PAAm, linear PAAm, does not obey a powerlaw since there G' and G'' intersect at approximately 100Hz. The CIRS phantom (matrix) showed almost perfect elastic behavior with μ = 5.82±0.20kPa and very low α of 0.02±0.01. The measured viscoelastic constants are summarized in table 1.Discussion
To the best of our knowledge, this study combines for the first time low-dynamic mechanical tests with high-frequency MRE at two different MRI systems in order to investigate the viscoelastic powerlaw behavior of generic gel samples over more than 11 octaves and three orders of magnitude frequency range. While heparin and crosslinked PAAm feature solid powerlaw behavior from 10 to 1200Hz and 1 to 3000Hz, respectively, linear PAAm has more fluid than solid properties up to 100Hz. In contrast, the CIRS-matrix is an almost perfect rubber-like solid without significant loss and cannot mimic biological soft tissue properties. Deviations from the powerlaw behavior at very low frequencies (< 1Hz) are expected since the springpot model does not support static stress. Ideally, a phantom material should obey perfect powerlaw behavior with an α-parameter similar to that observed in biological tissues. This requirement is best fulfilled for heparin in our study. However, heparin has softer properties than encountered in soft in-vivo tissues such as the liver or brain. On the contrary, crosslinked PAAm covers a similar range of stiffness as measured for in-vivo tissues, however, with lower dispersive properties. Nevertheless, both materials are good candidates for the use as standard phantom materials in MRE since both have well predictable springpot properties over the entire frequency range relevant for MRE investigations.Conclusion
We analyze heparin, PAAm polymer samples and a standardized CIRS elastography phantom by combining shear rheometry with MRE in a clinical system and a benchtop lab system. Dispersion functions in a wide dynamic range up to 11.8 octaves, were measured and analyzed by the springpot powerlaw model. Both, heparin and crosslinked PAAm feature solid powerlaw properties with suitable μ- and α-parameter ranges for comparison to soft biological matter.Acknowledgements
No acknowledgement found.References
1. Sack, I., et al., Structure-sensitive elastography: on the viscoelastic powerlaw behavior of in vivo human tissue in health and disease. Soft Matter 2013, 9(24), 5672-5680
2. Braun, J.,et al., A compact 0.5 T MR elastography device and its application for studying viscoelasticity changes in biological tissues during progressive formalin fixation. Magnetic Resonance in Medicine 2017.
3. Yasar TK, Royston TJ, Magin RL., Wideband MR elastography for viscoelasticity model identification. Magnetic resonance in medicine 2013;70(2):479-489.
Figures