2278
Validation of intrinsic actuation MR Elastography through a 1Hz experimental phantom system1Thayer School of Engineering, Dartmouth College, Hanover, NH, United States, 2Mechanical Engineering, Université de Sherbrooke, Sherbrooke, QC, Canada, 3Dartmouth Hitchcock Medical Center, Lebanon, NH, United States
Synopsis
A 1Hz MR elastography (MRE) phantom system is presented to validate the spatial accuracy of mechanical property images in intrinsic actuation MRE. A custom hydraulically driven actuator generated 1Hz shearing motions in gelatin phantoms with stiff inclusions which were measured using a retrospectively gated QFLOW sequence on a 3T Philips Achieva MRI. Maps of the octahedral shear strain showed low strain in stiff inclusions, and high strains in areas of stress concentrations, as expected from theory. Shear modulus maps computed by a viscoelastic nonlinear inversion MRE algorithm were spatially accurate, and identified the correct stiffness contrast of phantom components.
Introduction
MR elastography (MRE) has typically used 30-100Hz external vibrations to produce motion fields required to compute mechanical property images. We have previously demonstrated intrinsic actuation MRE (IA-MRE), which uses the natural pulsation of brain tissue during the cardiac cycle as a motion source1, and thus operates at a much lower frequency range ($$$\approx$$$ 1Hz). Repeatability of IA-MRE in healthy subjects of 2.6-9% for serial scans of the same subjects was reported, however, lack of a reliable, independent subject-specific stiffness measurement for living tissue leaves open questions about the quantitative and spatial accuracy of IA-MRE property images. An experimental, low-frequency phantom system where the stiffness distribution is known prior to imaging is required.Methods
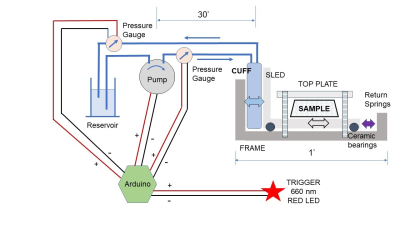
An actuator (figure 1) was constructed consisting of a sliding plate supported by ceramic bearings, pushed by an expandable blood pressure cuff. A fixed top plate is attached to the stationary outer tray, which supplies a preload to the phantom and ensures shearing motion between the top plate and sliding tray, as the actuation frequency is too low for free vibrations to produce enough strain to probe the shear modulus. The pressure in the cuff was actuated with an Arduino controller driving a small centrifugal pump, and a 30 ms light pulse from a red LED at the driving frequency of the pump was used as input to a pulse oximeter to trigger the MRI acquisition.
Phantoms were constructed from different gelatin components to produce stiff and soft inclusions. Relaxation rates similar to brain were achieved by adding 50-100 μM MnCl2.4H20 to the gelatin solution prior to cooling. Soft, intermediate and stiff gels were created from 3.5, 7 and 10.5 % gelatin solutions, heated to 75oC to dissolve, poured into molds after cooling to 35oC, then allowed to cool in the refrigerator. Phantoms with soft background were capped with intermediate stiffness gel to avoid damage at the surfaces contacting the actuator during long acquisitions.
Motion data was collected using a 3T Phillips Achieva MRI. Quantitative flow (QFLOW) acquisitions with 4mm isotropic voxels were executed along all three coordinate directions, with cardiac synchronization performed using retrospective gating at 1 Hz1 to produce velocity images at 16 phase offsets. The magnet was triggered for acquisition using the peripheral pulse unit (PPU).
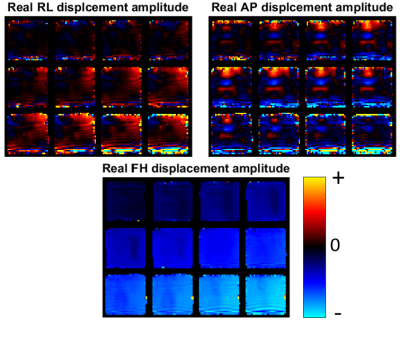
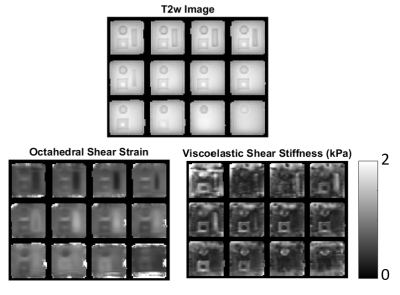
Velocity images were Fourier transformed across the 16 phase offsets to extract the 1Hz component, and Fourier integrated to obtain 3D maps of complex-valued displacement amplitudes. The octahedral shear strain (OSS) distribution2 was computed as an initial evaluation. A nonlinear inversion (NLI) property recovery algorithm3 was then applied to the data, assuming a nearly incompressible viscoelastic material, which is an appropriate model for gel phantoms.
Results
Results for an example phantom are shown in figures 3-4. The motion maps in figure 3 appear as expected, with the strongest component in the direction of the actuation (FH), and low motions near the top fixed plate. Some susceptibility artifact from interfaces is evident at the boundaries of the phantom. The OSS distribution in figure 4 clearly shows lower strain in stiffer inclusions, as well as the stress concentrations (high strain) around the inclusions that are expected in heterogeneous phantoms. Shear modulus maps from viscoelastic nonlinear inversion correctly identify the contrast between the stiff cylindrical inclusions, and the intermediate stiffness square prism with soft core. The edge susceptibility artifacts cause local errors in the property maps, however, the central part of the phantom is unaffected.
Discussion and Conclusions
This report is the first demonstration of a low frequency MR elastography phantom system, and the success of the IA-MRE imaging and inversion pipelines in this system supports the spatial accuracy of in vivo IA-MRE images. Shear modulus images with reasonably high spatial resolution were recovered from 4mm isotropic motion data, and the correct contrast between phantom components was recovered.
Future work with this phantom system will include a comprehensive evaluation of the achievable spatial resolution of each property type, experiments with poroelastic and viscoelastic materials/inversions at low frequencies, and examining the transition zone between intrinsic and external actuation MRE (between 2 and 20 Hz).
Acknowledgements
NIH Grant 1R01EB018230-01References
[1] Weaver, John B., et al. "Brain mechanical property measurement using MRE with intrinsic activation." Physics in medicine and biology 57.22 (2012): 7275.
[2] McGarry, M. D. J., et al. "An octahedral shear strain-based measure of SNR for 3D MR elastography." Physics in medicine and biology 56.13 (2011): N153.
[3] McGarry, M. D. J., et al. "Multiresolution MR elastography using nonlinear inversion." Medical physics 39.10 (2012): 6388-6396.
Figures