2275
Chemical Exchange Saturation Transfer (CEST) MRI of glucosamine at 3T1School of Chemistry, Tel Aviv University, Tel Aviv, Israel, 2Department of Neurobiology, Tel Aviv University, Tel Aviv, Israel
Synopsis
In our previous work using preclinical 7T MRI scanner we have shown that tumors in mice can be imaged using CEST-MRI of glucosamine. Moving toward clinical application, considering the excellent safety profile of glucosamine, we tested the CEST-MRI of glucosamine on a 3T clinical scanner. Here we report significant CEST MRI signal up to ~3.5 ppm from the water signal corresponding to the exchangeable protons of the glucosamine hydroxyls and amine residues. Thus, CEST MRI using glucosamine has the potential to report on the activity of tumor metabolism, noninvasively by using MRI.
INTRODUCTION
The efficacy of glucosamine (GlcN) as an agent for chemical exchange saturation transfer (CEST) MR molecular imaging of tumors was recently demonstrated in several animals' tumor models1. We suggest applying GlcN CEST-based MRI to develop novel molecular imaging modality for tumor and metastases detection and follow-up; the potential of clinical application of CEST MRI with GlcN is strengthened by its lack of toxicity as can be indicated from its wide use as food supplement2. Here, the translation of GlcN CEST MRI method to clinical MRI scanner was examined, in order to evaluate the feasibility of the new contrast agent to obtain new class of images.METHODS
In vitro experiments were performed on phantoms consisted of GlcN sulfate solutions (25-55 mM). MRI scans were conducted on a 3T Siemens Prisma clinical MRI scanner system. Images were acquired at room temperature using a 64-channel phased-array head coil for RF reception. The CEST protocol includes series of frequencies, using a train of 3-30 gauss saturation pulses with 50-100 ms long, interpulse delay of 22-61 ms and 2 s pause between measurements, saturation attenuations were in the range of 1.0-3.0 μT. The images were acquired using a single-shot turbo gradient echo with cubic resolution of 3 mm3. TR/TE/flip angle = 5.2 ms/2.7 ms/10o, resulting in a scan time under 3 minutes.RESULTS
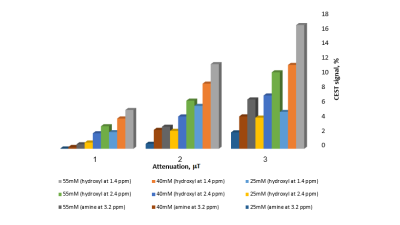
We demonstrated that GlcN can be detected by CEST- MRI at clinical scanner (3T). Several choices of irradiation parameters were tested, representative results are shown in figures 1 and 2. The MTRasym plot of 25-55 mM GlcN solutions showed significant signal in the hydroxyls regions over the entire saturation frequency offset range, the signal was proportional to the concentration (Fig.1). GlcN CEST signal was examined in terms of saturation pulse duration (Fig.1) as well as saturation attenuation (Fig. 2). As there is some overlap in GlcN signal when measured at different saturation pulse durations, the preferred parameters for the CEST experiment will be those that will give maximum signal with minimum transmission time (low SAR). The average magnitudes of CEST signal obtained with B1=3 μT and a total saturation pulse duration of 2.75 s were 2.6 and 1.8% per 10 mM at frequencies offsets of 1.4 and 2.4 ppm respectively corresponding to the exchangeable protons of the hydroxyls at the 3,4 and at the 1 positions, respectively. The CEST contrast achieved at ~3.2 ppm may be dominated by the amine protons transfer effect3. In that range, the average CEST signal was 1.1% per 10 mM of the agent.DISCUSSION
Initial phantom GlcN CEST MRI experiments at 3T have shown promising practical utility for evaluating cancer. GlcN generates a significant CEST MRI signal up to ~3.5 ppm from the water signal, likely arises from a mixture of GlcN hydroxyl protons groups and amine protons group. In tumor cells, the signal is expected to be higher owing to the contribution of GlcN metabolic products (that accumulate in the cells), thus GlcN CEST MRI signal has the potential to report on the activity of tumor metabolism. The amine protons of GlcN can produce clinical CEST MRI contrast with selective saturation at 3.2 ppm, even at the lower magnetic field strength (3T). Generally, the rate of chemical exchange of amine protons with water is relative fast and might be too fast to generate CEST at high pH (>7.0). But, an acidic tumor microenvironment of pH<7.0 may slows the chemical exchange of these protons, causing an increase in CEST contrast.CONCLUSIONS
These findings provide preliminary support for the potential use of GlcN as a new MRI contrast agent to detect tumors, tumor response to therapy and tumors metabolism, noninvasively by using MRI.Acknowledgements
No acknowledgement found.References
1. Rivlin, M, Navon, G. Glucosamine and N-acetyl glucosamine as new CEST MRI agents for molecular imaging of tumors. Scientific Reports Nature 2016; 6 (32648) doi:10.1038/srep32648.
2. Anderson, JW et al. Glucosamine effects in humans: a review of effects on glucose metabolism, side effects, safety considerations and efficacy. Food Chem Toxicol 2005;43(2):187-201.
3. Consuelo N, Beecher and Cynthia K. Larive, 1H and 15N NMR Characterization of the Amine Groups of Heparan Sulfate Related Glucosamine Monosaccharides in Aqueous Solution. Analytical Chemistry. 2015; 87(13): 6842-6848.
Figures