2272
Increased CEST specificity for amide and fast exchanging amine protons using exchange-dependent relaxation rate1Institute of Science and Technology for Brain Inspired Intelligence, Fudan University, Shanghai, China, 2Vanderbilt University Institute of Imaging Science, Vanderbilt University, Nashville, TN, United States
Synopsis
It is challenging to remove overlapping chemical exchange saturation transfer (CEST) signals from nearby exchanging sites. Our previous study showed that the contributions of fast exchanging amines to CEST signals at 3 ppm induce a broad spectral region that overlaps with the amide proton transfer (APT) spectrum centered around 3.5 ppm. In this work, we apply an exchange-dependent relaxation rate (Rex) for quantifying CEST effects to increased CEST specificity for amide and fast exchanging amine protons. Our results demonstrate that Rex reduces the influences of overlapping CEST signals for APT imaging, and thus can significantly enhances the CEST detection specificity.
Introduction
The quantification of chemical exchange saturation transfer (CEST) imaging is important for the analysis of in vivo CEST data. However, it is still challenging to remove overlapping CEST signals from nearby exchanging sites. Our previous study [1] showed that the contributions of fast exchanging amines to CEST signals at 3 ppm induce a broad spectral region that overlaps with the amide proton transfer (APT) spectrum centered around 3.5 ppm. In CEST imaging of fast exchanging amines, which is usually performed at relatively high irradiation powers (e.g. > 3 mT), the CEST peak shifts making it difficult to identify specific CEST effects [2]. In this work, we apply an exchange-dependent relaxation rate (Rex) for quantifying CEST effects to increased CEST specificity for amide and fast exchanging amine protons.Methods
In this work, we used magnetization transfer ratio (MTR), apparent exchange-dependent relaxation (AREX), exchange-dependent relaxation rate (Rex), and ΔRex (the subtraction of Rex acquired at a high irradiation power from that at a low irradiation power) for the quantification of CEST data.
MRI: five rats were imaged in this study. All measurements were performed using a Varian DirectDriveTM horizontal 9.4T magnet with a 38-mm Litz RF coil. CEST measurements were performed by applying a continuous wave irradiation with irradiation duration of 5 s and ω1 of 1 mT and 3.6 mT before acquisition. All images were obtained using a single-shot SE-EPI acquisition with triple references for phase correction and matrix size 64 × 64 and field of view 30 mm × 30 mm.
Data analysis: we used an extrapolated semi-solid MT reference (EMR) approach [3,4] to fit reference signals for quantifying CEST signals, and derived the metrics as MTREMR, AREXEMR, Rex_EMR, and ΔRex_EMR, respectively. Due to the relatively homogenous B1 field in rat brains, ω1 used to calculate the tangent theta were from nominal values. For animal studies, region of interests (ROIs) were chosen from the whole rat brains. All data analyses were performed using MATLAB 2013b (Mathworks, Natick, MA, USA).
Results
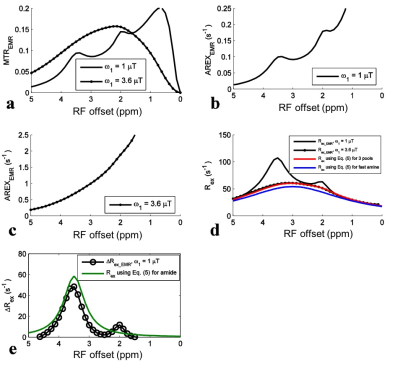
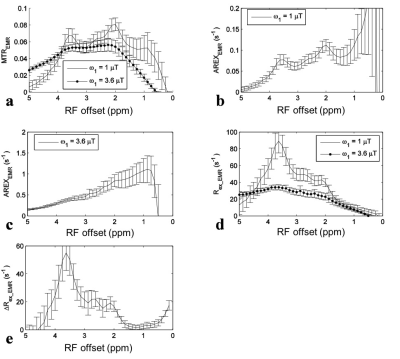
For simulations with ω1 of 1 mT, the APT signal at 3.5 ppm in the MTREMR spectrum (Fig. 1a) overlaps with nearby CEST signals. These nearby overlapping signals are still present in the AREXEMR spectrum (Fig. 1b). However, the intermediate exchanging amine signal at 2 ppm becomes relatively weak in the Rex_EMR spectrum (Fig. 1d) and the ΔRex_EMR spectrum (Fig. 1e). Fig. 1d also shows that the Rex_EMR spectrum with ω1 of 3.6 mT roughly matches the baseline of that with ω1 of 1 mT. Fig. 1e also shows the ΔRex_EMR spectrum in which both the nearby intermediate exchanging amine at 2 ppm and the fast exchanging amine at 3 ppm are successfully reduced. Fig. 2 shows experimental results from rat brains. Similar to the simulations in Fig. 1, the APT signal at 3.5 ppm acquired with ω1 of 1 mT overlaps with nearby CEST signals in the MTREMR spectrum (Fig. 2a), but can be successfully isolated from nearby CEST signals in the ΔRex_EMR spectrum (Fig. 2e). In addition, the CEST peak acquired with ω1 of 3.6 mT shows no distinct feature around its resonance frequency offset in the AREX spectrum (Fig. 2c), but shows a clear peak centered at around 3 - 4 ppm in the Rex_EMR spectrum (Fig. 2d). Fig. 3 shows maps of Rex_EMR at 3 ppm with ω1 of 3.6 mT (predominated by the contrast from fast exchanging amine) and ΔRex_EMR at 3.5 ppm (predominated by the contrast from amide) from a representative rat brain.Discussion
For quantitative CEST data analyses, it is important to estimate reference signals accurately in order to isolate the true chemical exchange effects from other confounding effects including MT and DS. Unfortunately, there is currently no perfect method for this purpose. In the current work, the EMR approach was used for fitting reference signals, but our analysis indicates that the EMR is still not robust. Note that the Rex method introduced here is not limited to the EMR method. Some other methods, such as Lorentzian fitting, may be combined with Rex as well to evaluate reference signals, although the accuracy of these methods at high powers has not been comprehensively investigated either. Further development of methods for obtaining more accurate reference signals may increase the in vivo application of Rex.Conclusion
We conclude that the Rex reduces the influences of overlapping CEST signals for APT imaging and provides Lorentzian lineshapes centered at their resonance frequencies for fast exchanging amines, and thus can significantly enhances the CEST detection specificity.Acknowledgements
No acknowledgement found.References
[1] Zhang XY, Wang F, Li H, et al. Accuracy in the quantification of chemical exchange saturation transfer (CEST) and relayed nuclear Overhauser enhancement (rNOE) saturation transfer effects. NMR Biomed. 2017 Jul;30(7). doi: 10.1002/nbm.3716.
[2] Cai KJ, Haris M, Singh A, et al. Magnetic resonance imaging of glutamate. Nat Medicine. 2012;18(2):302-306.
[3] Heo HY, Zhang Y, Lee DH, et al. Quantitative Assessment of Amide Proton Transfer (APT) and Nuclear Overhauser Enhancement (NOE) Imaging with Extrapolated Semi-Solid Magnetization Transfer Reference (EMR) Signals: Application to a Rat Glioma Model at 4.7 Tesla. Magn Reson Med. 2016;75(1):137-149.
[4] Heo HY, Zhang Y, Jiang SS, et al. Quantitative Assessment of Amide Proton Transfer (APT) and Nuclear Overhauser Enhancement (NOE) Imaging with Extrapolated Semisolid Magnetization Transfer Reference (EMR) Signals: II. Comparison of Three EMR Models and Application to Human Brain Glioma at 3 Tesla. Magn Reson Med. 2016;75(4):1630-1639.
Figures