2260
Simultaneous Multi-slice Rapid MR Elastography of the Liver1Radiology, The Ohio State University Wexner Medical Center, Columbus, OH, United States
Synopsis
We demonstrate the feasibility of combining simultaneous multi-slice (SMS) excitation with in-plane acceleration to achieve highly accelerated MR elastography data acquisition. The proposed approach enables the acquisition of diagnostic liver MRE data in a single breath-hold, which was not possible using the previous approaches. Our results indicate excellent agreement between the data acquired with and without SMS.
Introduction
Magnetic Resonance Elastography (MRE) is increasingly being utilized for the diagnosis and staging of liver fibrosis. Current clinical protocol for liver MRE data acquisition requires four ~18 second breath-holds to acquire 4-slice diagnostic gradient recalled echo (GRE) MRE data 1. Chamarthi et al. proposed a method (referred to as rapid MRE or MREr) to reduce the acquisition time by acquiring data with same motion encoding gradient (MEG) polarities during opposing phases of mechanical vibration 2. Although MREr results in shorter breath-holds, multiple breath-holds are still needed for acquisition of diagnostic MREr data. We propose incorporation of simultaneous multislice acquisition into MREr (SMS-MREr) to further reduce the acquisition time, thereby enabling the acquisition of the required images within one 16 second breath-hold. We demonstrate that SMS-MREr can be used to obtain diagnostic quality liver elastograms and report excellent agreement between the results obtained using SMS-MREr and MREr. The use of SMS without in-plane under-sampling to accelerate MRE acquisition has been reported previously 3. However, to our knowledge, this is the first study that utilizes SMS along with in-plane acceleration for MRE.
Methods
23 healthy volunteers were imaged on a 1.5T MRI scanner (Magnetom Aera, Siemens Medical Solutions Inc., Erlangen, Germany). A commercial pneumatic driver system was used to introduce 60 Hz mechanical vibrations in subjects’ liver (Resoundant, Mayo Clinic Foundation, Rochester, MN). We used MREr 2 and the developed SMS-MREr sequences to acquire MRE data. Briefly, MREr reduces the scan time (relative to the standard GRE based sequence) by playing MEGs with same polarity during opposing phases of mechanical vibration cycle. SMS-MREr further reduces the scan time by utilizing multiband excitation pulses. RF-based CAIPIRINHA was implemented to reduce g-factor penalty 4. The following imaging parameters were identical for both the MREr and SMS-MREr sequences: TE = 21.4 ms; TR =25 ms; FOV = 360 mm; Flip angle 20°; slice thickness = 5 mm; 4 slices; matrix = 128x64; vibration frequency = 60Hz; 4 MRE time offsets; MEG of 16.67 ms duration (60Hz) along the slice direction. Generalized Autocalibrating Partially Parallel Acquisitions (GRAPPA) acceleration factor of 2 with 16 reference lines was utilized in MREr to reduce the breath-hold duration per slice to 8 seconds. For SMS-MREr, we used GRAPPA acceleration factor of two with 8 reference lines which were not used in the reconstruction. This resulted in 14 second acquisition for 4 slices. A two second long low resolution GRE based reference scan was acquired immediately before SMS-MREr acquisition to serve as a reference scan for split slice-GRAPPA reconstruction 5, resulting in a total breath-hold duration of 16 seconds for the acquisition of 4 slices. Images were constructed using custom developed MATLAB scripts. Stiffness maps were generated using MRE Lab (Mayo Clinic, Rochester, MN). Identical ROIs were manually drawn on SMS-MREr and MREr derived stiffness maps to obtain average stiffness values.Results
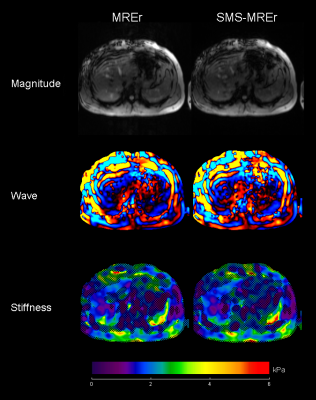
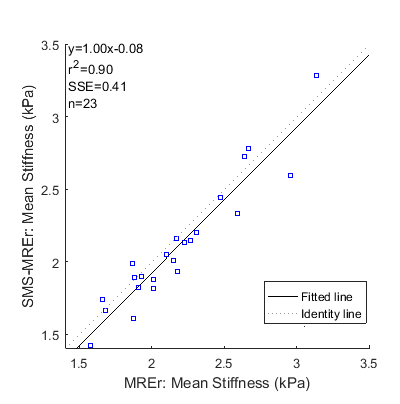
Magnitude, wave snapshot and stiffness images from volunteers with elevated and normal liver stiffness are shown in Figures 1 and 2 respectively. Visual inspection suggests that the simultaneously acquired slices were successfully separated using the split slice-GRAPPA algorithm. Magnitude, wave and stiffness images acquired using both methods show excellent visual agreement. Indeed, excellent linear relationship between the mean stiffness values obtained using MREr and SMS-MREr is observed (Figure 3), albeit with a slight negative bias in SMS-MREr (p-value = 0.014). Additionally, Bland-Altman analysis demonstrates excellent agreement between the stiffness values obtained using MREr and SMS-MREr for all subjects except one. Maximum difference of 0.36kPa was observed between MREr and SMS-MREr derived stiffness values.Discussion and Conclusions
In this study, we demonstrate, for the first time, that it is possible to combine SMS and in-plane acceleration for diagnostic MR elastography. This, combined with the rMRE approach, enables diagnostic MRE data acquisition with unprecedented speed. The stiffness values obtained with MREr and SMS-MREr exhibit agreement in general. The negative bias observed for SMS-rMRE may be caused by the reduction in SNR due to g-factor penalty, since an inter-slice gap of 0mm was used in this study. The inherent variation between breath-holds results in variation in the orientations of the slices acquired during multiple breath-holds. Also, the inter-slice gap may not be uniform across the slices. The proposed approach overcomes these limitations on the anatomical precision by enabling the acquisition within a single breath-hold. Future work will focus on further improving the SMS-rMRE image quality by optimizing sequence parameters and RF profile.
Acknowledgements
Authors would like to thank NIH-NHLBI for grant support (R01HL124096).References
- Dzyubak, B., et al. (2016). "Automated liver elasticity calculation for MR elastography." Journal of Magnetic Resonance Imaging 43(5): 1055-1063.
- Chamarthi, S. K., et al. (2014). "Rapid acquisition technique for MR elastography of the liver." Magnetic resonance imaging 32(6): 679-683.
- Guenthner, C., et al. (2017). "Simultaneous Multislice Acquisition for Magnetic Resonance Elastography." Proceedings of 25th ISMRM Annual Meeting: Abstract 1137
- Breuer, F. A., et al. (2005). "Controlled aliasing in parallel imaging results in higher acceleration (CAIPIRINHA) for multi‐slice imaging." Magnetic resonance in medicine 53(3): 684-691.
- Cauley, S. F., et al. (2014). "Interslice leakage artifact reduction technique for simultaneous multislice acquisitions." Magnetic resonance in medicine 72(1): 93-102.
Figures