2253
Longitudinal Stability of a Quantitative Fat-Water Phantom1Biomedical Engineering, University of Wisconsin, Madison, Madison, WI, United States, 2Radiology, University of Wisconsin, Madison, Madison, WI, United States, 3Medical Physics, University of Wisconsin, Madison, Madison, WI, United States, 4Medicine, University of Wisconsin, Madison, Madison, WI, United States, 5Emergency Medicine, University of Wisconsin, Madison, Madison, WI, United States
Synopsis
The purpose of this work was to evaluate the long-term stability of a previously validated fat-water phantom under a range of environmental conditions. Two separate phantoms were constructed, each with a range of fat concentrations. The first phantom was subjected to three different temperature conditions over one year. The second fat phantom was kept at room temperature and studied over three years. Our results show that the fat-water phantom has excellent long-term stability at room temperature and is robust to different temperature conditions.
Introduction
Chemical shift encoded (CSE)-MRI has shown tremendous promise for quantification of proton-density fat-fraction (PDFF) in multiple applications. Phantoms play an important role in the development, validation, and quality assurance of CSE-MRI methods1,2. Recently developed fat-water phantoms enable single-site1,3,4 and multi-site2 validation of CSE-MRI methods. However, the long-term stability of current fat-water phantoms is unknown. Long-term stability is critical for longitudinal quality assurance for clinical and research applications. Therefore, the purpose of this work is to evaluate the long-term stability of a previously validated fat-water phantom under a range of environmental conditions.Materials and Methods
Phantom Construction:
Two fat-water phantoms, consisting of agar-based oil-water emulsions, were constructed as previously described1,2. The first phantom consisted of four vials with PDFF=0%, 15%, 30%, and 100%. The cap of each vial was lined using silicone gel for improved sealing, and a small quantity of petroleum jelly was layered on the top of each emulsion. This phantom was constructed in 04/2016.
Additionally, a previously constructed phantom, which had been employed in a multisite study2 between 10/2014 and 12/2015, was scanned periodically in order to assess longer-term stability. This phantom included 11 vials with PDFF=0%, 2.6%, 5.3%, 7.9%, 10.5%, 15.7%, 20.9%, 31.2%, 41.3%, 51.4%, and 100%.
Environmental Conditions:
Three identical replicates of the first phantom were constructed and stored for one year, under the following conditions. One replicate was stored at room temperature, a second in a refrigerator at approximately 2°C, and the third within a contrast-warming oven at 38°C. The second phantom was stored at room temperature for three years.
MR Imaging:
On a periodic basis, each phantom was removed from its environment and placed in the scan room approximately 60 minutes prior to scanning, to allow temperature equilibration. All imaging was performed at 1.5T (HDxt, GE Healthcare, Waukesha, WI) using a single-channel quadrature head coil. CSE-MRI was performed using a 3D spoiled gradient echo acquisition, with parameters: FOV = 300mm x 240mm, 6 echoes within one TR, TR=16.4 ms, TE1=1.2 ms/ ΔTE= 2.0 ms, BW= ±100 kHz, 4 mm slices, 20 slices, 5° flip angle. No parallel imaging was used. Total scan time was 2:42 minutes. The same acquisition protocol was used for all imaging.
Confounder-corrected PDFF maps were reconstructed using an offline algorithm that included correction for R2* effects5,6 and the spectral complexity of fat5,6.
PDFF was measured from a central slice within each vial, with ROI size chosen to include the majority of the vial, avoiding partial volume effects at the edge of the vial.
Statistical analysis:
For each vial within each phantom, the slope of the measured PDFF over time (to assess systematic drift), as well as the standard deviation of PDFF measurements across the entire study (to assess overall variability), were calculated including 95% confidence intervals.
Results
The longitudinally acquired PDFF values were plotted for each of the three replicates of the first phantom (Figure 1). The 15% PDFF vial stored in the refrigerator was accidentally dropped on day 80 and was subsequently unusable. Statistical analysis demonstrated PDFF slope not significantly different from zero for the room temperature vials, while 1 of the 4 vials stored in the refrigerator had a statistically significant slope of -1.1%/year, and 2 of the 4 vials stored in the oven had a statistically significant slope, with maximum slope of +6.5%/year. Across all the vials in each replicate, the maximum standard deviation (upper limit of the 95% CI) in PDFF measurement was 2.88% (room temperature), 1.97% (refrigerator), and 3.91% (oven).
Figure 2 plots the PDFF measurements from the second phantom over a three-year period. Statistical analysis demonstrated no significant PDFF slope for 9 of the 11 vials during this time, whereas the remaining two vials had slopes below +0.5%/year. The maximum standard deviation for this phantom was 1.5%.
Discussion and Conclusion
In summary, we have successfully performed a longitudinal study of the stability of fat-water phantoms stored in three different environmental conditions over a period of 1-3 years. Highly consistent and stable PDFF measurements were observed over time for phantoms stored at room or refrigerator temperatures, whereas several vials stored at high temperatures (38°C) experienced PDFF drift over a 1-year period. Nevertheless, the drift observed at high temperatures was relatively slow (<6.5%/year, or equivalently <0.018%/day), suggesting that brief periods at high temperature (eg: as needed for shipment) will not have a substantial impact on the properties of the phantom. In conclusion, the fat-water phantom has excellent long-term stability at room temperature and is robust to high and low temperatures.Acknowledgements
The authors wish to thank the Discovery to Product Program at the University of Wisconsin-Madison for supporting this project. The authors also wish to thank GE Healthcare who provides research support to the University of Wisconsin, as well as the NIH for their support (R01 DK100651, K24 DK102595, R01 DK083380, R01 DK088925). SDS contributed to this work while at UW-Madison. SDS is now an employee of Toshiba Medical Research Institute, USA.References
1. Hines CD, Yu H, Shimakawa A, McKenzie CA, Brittain JH, and Reeder SB. T1independent, T2* corrected MRI with accurate spectral modeling for quantification of fat: Validation in a fat-water-SPIO phantom. J. Magn. Reson. Imaging 2009, 30: 1215–1222.
2. Hernando D, Sharma SD, Aliyari Ghasabeh M, Alvis BD, Arora SS, Hamilton G, Pan L, Shaffer JM, Sofue K, Szeverenyi NM, Welch EB, Yuan Q, Bashir MR, Kamel IR, Rice MJ, Sirlin CB, Yokoo T, and Reeder SB. Multisite, multivendor validation of the accuracy and reproducibility of proton-density fat-fraction quantification at 1.5T and 3T using a fat–water phantom. Magn. Reson. Med. 2017, 77: 1516–1524.
3. Hernando D, Hines CDG, Yu H, Reeder SB. Addressing Phase Errors in Fat-Water Imaging Using a Mixed Magnitude/Complex Fitting Method. Magn. Reson. Med. 2012, 67(3):638-644.
4. Bernard CP, Liney GP, Manton DJ, Turnbull LW, and Langton CM. Comparison of fat quantification methods: A phantom study at 3.0T. J. Magn. Reson. Imaging 2008, 27: 192–197.
5. Hernando D, Kellman P, Haldar JP, and Liang ZP. Robust water/fat separation in the presence of large field inhomogeneities using a graph cut algorithm. Magn. Reson. Med. 2010, 63: 79–90.
6. Yu H, Shimakawa A, McKenzie CA, Brodsky E, Brittain JH, and Reeder SB. Multiecho water-fat separation and simultaneous R estimation with multifrequency fat spectrum modeling. Magn. Reson. Med. 2008, 60: 1122–1134.
Figures
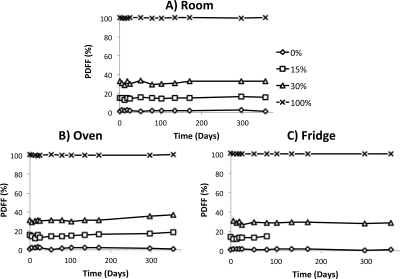
Figure 1: Temperature Phantom Data
The plot shows the temperature phantom PDFF measurements over the time span that the phantoms were scanned and studied for the three conditions. Statistical analysis demonstrated no significant change in PDFF for the room vials, while 1 of the 4 fridge vials (1.1% decrease/year) and 2 of the 4 oven (max of 6.5% increase/year) vials were statistically significant. The 15% PDFF vial stored in the refrigerator was accidentally dropped on day 80 and was subsequently unusable.
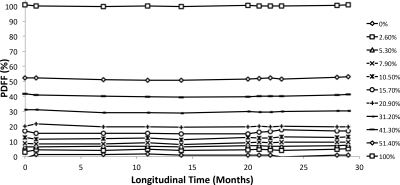
Figure 2: Multi-Site Phantom Data
The plot shows the multi-site phantom PDFF measurements over the time span that the phantoms were scanned and studied. Overall, 9 of the 11 vials demonstrated no statistically significant change over this time, while the remaining two had slopes below 0.5% increase/year.