2242
Implications of tissue compartmentalisation on APT MRI1Wellcome Centre for Integrative Neuroimaging, FMRIB, Nuffield Department of Clinical Neurosciences, University of Oxford, Oxford, United Kingdom, 2CRUK & MRC Oxford Institute for Radiation Oncology, University of Oxford, Oxford, United Kingdom, 3Institute of Biomedical Engineering, University of Oxford, Oxford, United Kingdom
Synopsis
Amide proton transfer (APT) MRI is assumed to report on the intracellular environment. However, no attempt has been made to verify this assumption, or examine the extent to which tissue compartmentalisation contaminates measurements of biophysical parameters (e.g. protein concentration, pH) from APT MRI data. In this simulation study, we show that measurement of biophysical parameters by APT MRI is influenced by tissue compartmentalisation when the transcytolemmal exchange rate is slow (<2Hz). Since recent studies have reported transcytolemmal exchange to be in such a regime, it may be possible to separate intra- and extracellular APT signals.
Introduction
Amide proton transfer (APT) MRI is assumed to report on the intracellular environment because the APT signal originates from amide protons on molecules in a sufficiently ‘mobile’ environment[1]. However, no attempt has been made to verify this assumption, or to examine the extent to which tissue compartmentalisation contaminates measurements of biophysical parameters (e.g. protein concentration, pH) from APT MRI data. Contamination of these measurements will be exacerbated in pathologies where the intra- and extracellular environments vary markedly, such as tumours[2]. Isolated measurement of the APT signal from either compartment would be advantageous; however, transcytolemmal exchange of intra- and extracellular water may be too fast to allow this.
In this simulation study, the effect of tissue compartmentalisation on APT MRI is investigated, with the aim of determining whether separated intra- and extracellular Z-spectra can be recorded, thus enabling more specific measurement of biophysical parameters in vivo.
Methods
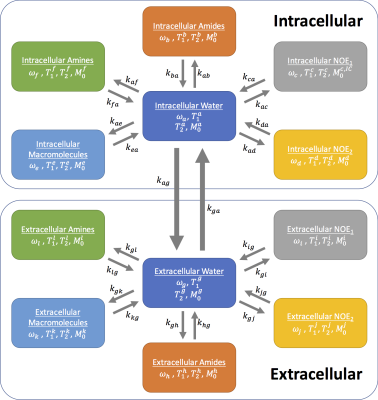
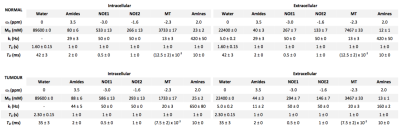
Z-spectra from intra- and extracellular tissue compartments for normal and tumour tissue were simulated using the Bloch-McConnell equations. Two six-pool systems representing the intra- and extracellular tissue were simulated with a transcytolemmal exchange term between the two systems (kcyto). A schematic of the exchange model is shown in Figure 1, as well as parameters for each tissue type in Figure 2, where tumour pHe is more acidic and intracellular amide concentration elevated compared to normal tissue. Each parameter was assumed to be normally distributed with a mean and standard deviation as shown in Figure 2. 200 instances of the simulations were run with random selection of each parameter from its respective distribution to reflect physiological variation; white noise was added to reflect measurement noise. The simulated CEST sequence comprised continuous wave saturation with B1=1.17μT for 2s and B0=9.4T. The overall water pool Z-magnetisation (i.e. MZ=MZA+MZG) was used for plotting Z-spectra and model fitting to reflect the non-specific measurement made in APT MRI.
Z-spectra were analysed using BayCEST in FSL, and the APT effect quantified using APTR*[3,4]. Further simulations were run to evaluate the contribution of each parameter that differed between tumour and normal tissue to the overall APTR* signal difference by varying each one in turn, with all other parameters kept at their normal tissue values. The signal difference was evaluated by calculating the ratio of APTR* with respect to normal tissue (rAPTR*). Finally, simulations with no physiological or measurement noise were run for normal and tumour tissue with variable kcyto to determine how it affects the measured APTR*.
Results
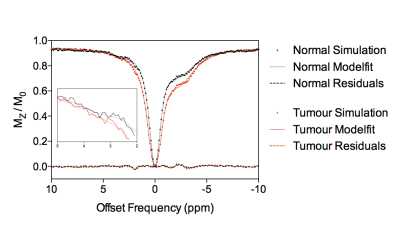
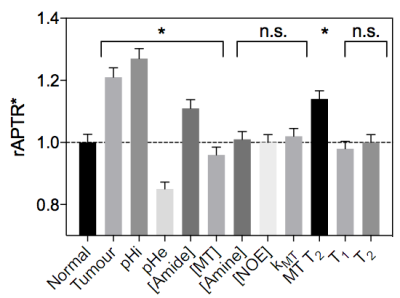
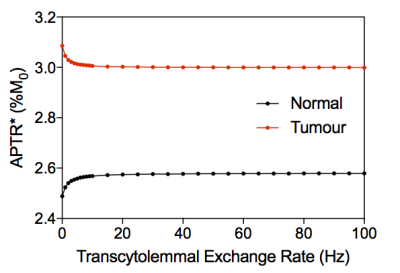
Figure 3 shows example simulated Z-spectra for normal and tumour tissue, along with the model fitted Z-spectra from the BayCEST analysis and the fitting residual. Figure 4 shows the mean ± 95% confidence interval rAPTR* for normal and tumour tissue, as well as the contribution of each parameter that differed between normal and tumour tissue to the overall APTR* difference. Deterministic simulation of normal and tumour tissue whilst varying the transcytolemmal exchange rate (kcyto), revealed that there is a small variation in APTR* when kcyto<10Hz, whereas APTR* is constant at a tissue-dependent plateau level when kcyto>10Hz (Figure 5).Discussion
Figure 4 suggests that pHi, pHe, amide and MT proton concentration, and T2 time of MT protons are the dominant factors determining the APTR* difference between normal and tumour tissue. The acidic pHe in tumours reduces APTR*, counteracting the effect of increased intracellular protein concentration and more alkaline pHi. The accuracy of any pH or protein concentration measurements made using APT MRI are likely to be contaminated by partial volume effects between tissue compartments within a voxel.
Figure 5 suggests that when kcyto>10Hz, the mixing of compartments makes separation of intra- and extracellular Z-spectra impossible. The physiological range of kcyto, however, is up to 10Hz[5]. In addition, the effect of the acidic pHe in the tumour acts to reduce APTR* as kcyto increases upto 10Hz.
One previous study[6] concluded that the influence of separate tissue compartments is negligible when kcyto>2Hz and the CEST saturation duration was on the order of seconds. However, other studies have measured kcyto to be 1.81Hz in in vivo brain[7], and 1.30Hz or 1.75Hz in cell suspension of neurons or astrocytes, respectively[8]. The results presented here suggest that since kcyto is in the <2Hz regime, tissue compartmentalisation has an influence on measured APT signals, and separate intra- and extracellular APT signals may be observable, although novel acquisition strategies would be required[9].
Conclusion
This simulation study showed that APT MRI signals (and any subsequent measurements of biophysical parameters) are likely influenced by tissue compartmentalisation if kcyto is slow (<2Hz). Since recent studies have reported kcyto to be in this regime, separation of intra- and extracellular APT signals using novel acquisition strategies may be possible.Acknowledgements
Funding was provided by a Medical Research Council studentship, the EPSRC & CRUK Cancer Imaging Centre - Oxford, and the Oxford NIHR Biomedical Research Centre. The Wellcome Centre for Integrative Neuroimaging is supported by core funding from the Wellcome Trust.References
[1] Zhou, J, Payen, J.F., Wilson, D.A., et al. "Using the amide proton signals of intracellular proteins and peptides to detect pH effects in MRI" Nat. Med. 2003; 9(8):1085-1090
[2] Hanahan, D., Weinberg, R.A. "Hallmarks of Cancer: The next generation" Cell 2011; 144(5):646-674
[3] Chappell, M.A., Donahue, M.J., Tee, Y.K., et al. "Quantitative Bayesian model-based analysis of amide proton transfer MRI" Magn. Reson. Med. 2013; 70(2):556-567
[4] Ray, K.J., Larkin, J.R., Tee, Y.K., et al. "Determination of an optimally sensitive and specific chemical exchange saturation transfer MRI quantification metric in relevant biological phantoms" NMR Biomed. 2016; 29(11):1624-1633
[5] Tian X., Li H., Jiang X., et al. "Evaluation and comparison of diffusion MR methods for measuring apparent transcytolemmal water exchange rate constant" J Magn. Reson. 2017; 275; 29-37
[6] Li, H., Li, K., Zhang, X.Y., et al. "R1 correction in amide proton transfer imaging: Indication of the influence of transcytolemmal water exchange on CEST measurements" NMR Biomed. 2015; 28(12):1655-1662
[7] Quirk, J.D., Bretthorst, G.L., Duong, T.Q., et al. "Equilibrium water exchange between the intra- and extracellular spaces of mammalian brain" Magn. Reson. Med. 2003; 50(3):493-499
[8] Yang D.M., Huettner J.E., Bretthorst G.L. et al. "Intracellular water preexchange lifetime in neurons and astrocytes" Magn. Reson. Med. 2017; ePub ahead of print
[9] Ray, K.J., Karunanithy, G., Baldwin, A.J. et al. "Separation of intracellular and extracellular Z-spectra by DiffusionCEST" Proc. Intl. Soc. Magn. Reson. Med. 2016; #0302
Figures