2232
CEST Feasibility in Rectal Cancer Patients at 7T for Detection of Residual Tumor1Imaging, University Medical Center Utrecht, Utrecht, Netherlands, 2Radiation Oncology, University Medical Center Utrecht, Utrecht, Netherlands, 3Utrecht University, Utrecht, Netherlands
Synopsis
Five patients were scanned at a 7T MR scanner, 9 weeks after chemoradiation treatment. Three patients showed extreme artifacts on the calculated CEST maps due to B0 artifacts from air contained in the rectum or poor B0 shimming. Two successful CEST measurements showed matching amide-CEST maps to the residual tumor observed in the MRI. CEST applied to rectum patients after chemoradiation might be the appropriate technique to avoid surgical resection in some patients without residual tumor after treatment.
Introduction
A wait and see approach is an option offered to patients with a complete clinical response when treated with chemoradiation for locally advanced rectal cancer1. 15 to 20% of these patients show complete pathological response at postoperative histological examination2 for whom surgical resection is questionable. However, the identification of complete responders on conventional imaging can be challenging as small residual tumor can be missed or misinterpreted as radiation induced fibrosis. The use of ultra-high fields, such as 7 Tesla (7T) might be useful for discrimination between tumorous and fibrous tissue. Higher field strength offers increased signal and contrast to noise (SNR, CNR), and increased spatial and spectral resolutions. With the increased spectral resolution, other contrast mechanisms, such as chemical exchange by saturation transfer (CEST) can be explored for the differentiation between tumor and healthy or non-tumorous rectal tissue. In particular, the amide-CEST and nuclear Overhauser effected (NOE) part of the Z-spectra have already shown altered levels in tumor when compared to normal tissue3. CEST is sensitive for pH changes, which leads to altered chemical exchange rates in amino acids with water hydroxyl protons. Tumor cells have a lower pH environment that may lead to different contrast between tumor and non-tumorous rectal tissue, which can be detected with this technique. Therefore, we developed a CEST technique and used it in patients with rectal cancer to test the viability of CEST for the detection of residual tumor after chemoradiation.Methods
five patients were scanned at a 7T MR scanner (Philips, Cleveland, USA) after giving informed consent. Eight transceiver fractionated dipole antennas4 with 16 additional receive loops (MR Coils BV, Drunen, The Netherlands) interfaced to eight-parallel 2kW channels were positioned symmetrically around the pelvis. RF phase shimming was performed to maximize and homogenize the B1+ field in the rectum region. The CEST protocol (2D fast gradient echo, TE/TR=3.6/15 ms, 46 saturation frequency offsets (-1500 to 1500 ppm), 80 Gaussian saturation pulses of 25 ms duration, effectively 1.3μT and a 30 ms interval; 220x4x393mm FOV, 4x4x4mm voxel, 5 min 42 sec acquisition time) was planned on a T2-weighted MRI. Data analysis was performed with home-built MATLAB (R2014b, The Mathworks, Inc. ©) scripts that include Lorentzian fitting of water, amides, amines and NOEs. Pixel and regions of interest (ROI) analysis were obtained.Results and Discussion
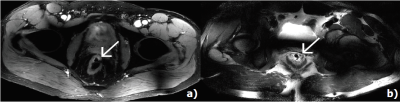
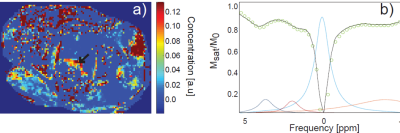
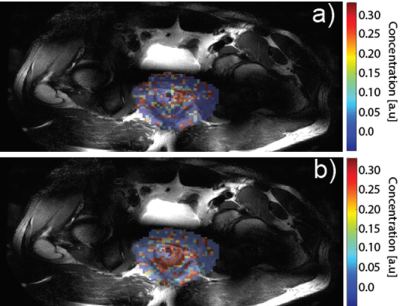
Figure 1 a) shows a DIXON water image (upper left corner) used to plan the CEST slice on a patient 9 weeks after chemoradiation. The calculated amide-CEST map shows increased signal (arrows) in part of the rectum. This patient was a partial responder (i.e. residual tumor tissue left after chemoradiation). Figure 1b) shows the signal for one voxel with the fitted amide (blue), amine (red), water (light blue) and NOE (orange) CEST effects. The increased spectral dispersion available at 7T enabled to obtain individual amide- and amine-CEST maps (figure 2 a),b) respectively). At lower field strengths, both resonances (3.5 ppm and 2.1 ppm respectively) would overlap. Figure 2 shows the CEST maps for a patient with a distal rectal tumor. Notice that the amine only covers half of the rectum. While the amide covers mostly the remaining tumor. NOE-CEST effects were overall low or not existing. Results for the first three patients were strongly affected by artifacts (not shown) arising from the air contained in the rectum and poor B0 shimming. Despite air cavities in the rectum, CEST maps of the rectum were feasible and increased signal intensities match to the observed anatomy on the T2 weighted MRI.Conclusions
Selective detection of amide- and amine-CEST in the rectum is feasible at 7T for rectal cancer patients in combination with a multi-transmit system. Amide-CEST maps may correlate with residual tumor, which might help to detect residual tumor after chemoradiation. Further research in a larger rectal cancer population is needed for verification.Acknowledgements
This research was possible thanks to the Maag Lever Darm Stichting, The Netherlands.References
[1] Glynne-Jones R, Hughes R. Critical appraisal of the 'wait and see' approach in rectal cancer for clinical complete responders after chemoradiation. The British journal of surgery. 2012 Jul;99(7):897-909. PubMed PMID: 22539154.
[2] Sauer R, Becker H, Hohenberger W, Rodel C, Wittekind C, Fietkau R, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. The New England journal of medicine. 2004;351(17):1731-40.
[3] Zhou IY, Wang E, Cheung JS, Zhang X, Fulci G, Sun PZ. Quantitative chemical exchange saturation transfer (CEST) MRI of glioma using Image Downsampling Expedited Adaptive Least-squares (IDEAL) fitting. Scientific Reports. 2017;7(1):84.
[4] Raaijmakers, A.J.E., et al., The fractionated dipole antenna: A new antenna for body imaging at 7 Tesla. Magnetic Resonance in Medicine, 2015.
Figures