2230
Multiple Interleaved Mode Saturation (MIMOSA) for B1+ inhomogeneity mitigation in chemical exchange saturation transfer.1Institute of Radiology, University Hospital Erlangen, Friedrich-Alexander-Universität Erlangen-Nürnberg (FAU), Erlangen, Germany, 2Max Planck Institute for Biological Cybernetics, Tuebingen, Germany, 3Siemens Healthcare GmbH, Erlangen, Germany, 4Siemens Healthcare Pty Ltd, Sydney, Australia
Synopsis
Due to high sensitivity to B1+-inhomogeneities, Chemical Exchange Saturation Transfer MRI requires a correction or mitigation of the B1+-inhomogeneity at ultra-high magnetic field strengths (B0 ≥ 7 Tesla). A novel approach for mitigation of B1+-inhomogeneity effects that affects the saturation process is presented. The method employs two interleaved excitation modes during the saturation pulse train. Simulations show a decrease of the relative difference of the MTRRex metric caused by B1+ inhomogeneity. This “Multiple Interleaved Mode Saturation” scheme leads to improved homogeneity in both, phantom and in vivo measurements at 7 Tesla.
Introduction
To benefit from the increased spectral resolution and higher SNR at high B0 (e.g. 7 Tesla), correction1-3 or mitigation4 of the transmit (B1+) inhomogeneity is required for CEST imaging. In this work, we evaluate a novel approach that is similar to the Time Interleaved Acquisition of Modes (TIAMO)5 method. The new method called Multiple Interleaved Mode Saturation (MIMOSA), exploits a parallel transmit (pTx) system to interleave two excitation modes during the saturation scheme.Methods
Numerical Bloch-McConnell simulations6, 7 have been performed with the use of MATLAB (The MathWorks, Natick, MA, USA). Pulse sequences were implemented on a 7-Tesla whole-body MR system (MAGNETOM Terra, Siemens Healthcare GmbH, Erlangen, Germany). An 8Tx/32Rx head coil (Nova Medical, Wilmington, USA) was used for the measurements. Phantom measurements have been performed with an 18 cm diameter spherical phantom filled with 50 mM Creatine and 2% Agarose gel with a pH of 7.0. In vivo measurements have been performed on a healthy male volunteer.
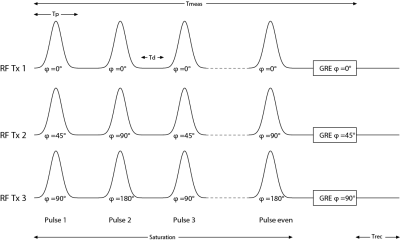
For both, phantom and in vivo measurements images were acquired using a single slice GRE sequence with pulsed CEST presaturation8, 9. In addition, B1+ maps were acquired using pre-saturated 2D turbo-flash sequence sequence. For the MIMOSA acquisition, two sets of transmitter phases with constant amplitudes were used for the saturation pulse train. The first mode has 45° phase difference between two adjacent transmitter coil channels (“Circular Polarization - CP”). In the second set a 90° phase increment between the transmitter coil elements has been chosen (90° mode). Figure 1 exemplarily shows for the first three Tx channels how the two modes have been interleaved during the saturation pulse train. Each mode has a different B1+ distribution. Thus, the interleaved saturation pulses will have two different effective B1+ values in a single voxel, in the following denoted as B1,A and B1,B. The GRE image acquisition has been performed with the use of the CP mode. For comparison CEST images were also acquired using a CP mode in both, acquisition and saturation scheme, which is equivalent to the use of a single transmitter (1Tx) system .
Sequence parameters: CEST Saturation parameters for phantom measurements: Tp = 99.84 ms, TD = 50 ms, n= 26, TRecovery = 3 s, B1,Nominal=0.4µT, Gaussian pulse shape; Saturation parameters for in vivo measurements: Tp = 15.32 ms, TD = 10 ms, number of pulses n=150, TRecovery = 0 s, B1,Nominal=0.6µT Gaussian pulse shape; GRE acquisition parameters: TR = 7.2 ms, TE = 3.45 ms, Matrix Size = 128x128, FoVphantom=190mm, FoVinvivo=210 mm;
Results and Discussion
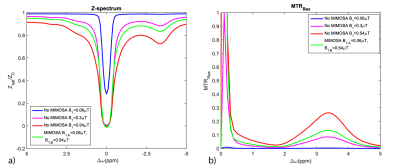
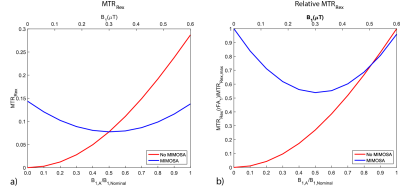
Numeric simulations showing the influence of MIMOSA on the Z-spectrum and MTRRex metric are presented in figure 2 and 3. Figure 2 shows a rise of the MTRRex metric in comparison to a simulation without MIMOSA at B1=0.3µT. Figure 3 shows that MIMOSA reduces the variation of the MTRRex metric caused by B1+-inhomogeneity in comparison to the standard saturation using CP mode. Figure 3 a) shows also an increase of the MTRRex metric in comparison to the standard method with 0.3 µT.
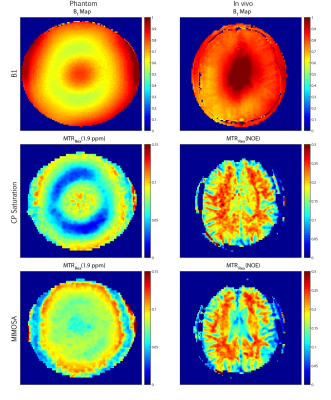
Measurement results from both phantom and brain acquisitions without and with the use of the MIMOSA are presented in figure 4. A correlation between the loss of MTRRex magnitude and the B1 map under saturation with CP mode in the phantom can be observed. The application of MIMOSA in phantom resulted in a more homogeneous image with still some slight loss of the MTRRex magnitude around areas affected by the B1+ inhomogeneity. The in vivo measurements presented in figure 3 show similar MTRRex values in the lateral ventricles as in white matter in case of the use of CP saturation. In the MIMOSA acquisition a decrease of the MTRRex magnitude can be observed in the lateral ventricles resulting in a stronger contrast between the ventricles and white matter.
Conclusion
A new approach to reduce signal variation due to B1+-inhomogeneity in CEST MRI has been proposed and analyzed. Two excitation modes during the saturation scheme have been applied. In comparison to previously employed pTx techniques4 it does not require an additional pulse optimization process. Although, the inhomogeneity could not be fully mitigated, simulations proved that with MIMOSA the MTRRex contrast can be acquired with a smaller relative difference in comparison to a single mode saturation. Images obtained with the use MIMOSA resulted in a more homogeneous MTRRex map both in phantom and in in vivo measurements.Acknowledgements
No acknowledgement found.References
1. Khlebnikov, V., et al., On the transmit field inhomogeneity correction of relaxation-compensated amide and NOE CEST effects at 7 T. NMR Biomed, 2017. 30(5).
2. Windschuh, J., et al., Correction of B1-inhomogeneities for relaxation-compensated CEST imaging at 7 T. NMR Biomed, 2015. 28(5): p. 529-37.
3. Schuenke, P., et al., Simultaneous mapping of water shift and B1 (WASABI)-Application to field-Inhomogeneity correction of CEST MRI data. Magn Reson Med, 2016.
4. Tse, D.H., et al., B1+ inhomogeneity mitigation in CEST using parallel transmission. Magn Reson Med, 2017.
5. Orzada, S., et al., RF excitation using time interleaved acquisition of modes (TIAMO) to address B1 inhomogeneity in high-field MRI. Magn Reson Med, 2010. 64(2): p. 327-33.
6. Woessner, D.E., et al., Numerical solution of the Bloch equations provides insights into the optimum design of PARACEST agents for MRI. Magn Reson Med, 2005. 53(4): p. 790-9.
7. Zaiss, M., et al., A combined analytical solution for chemical exchange saturation transfer and semi-solid magnetization transfer. NMR Biomed, 2015. 28(2): p. 217-30.
8. Zaiss, M., et al., Relaxation-compensated CEST-MRI of the human brain at 7T: Unbiased insight into NOE and amide signal changes in human glioblastoma. Neuroimage, 2015. 112: p. 180-8.
9. Schmitt, B., et al., Optimization of pulse train presaturation for CEST imaging in clinical scanners. Magn Reson Med, 2011. 65(6): p. 1620-9.
Figures