2220
Quantitative Susceptibility Mapping at high and ultra-high field: a reproducibility study1IMT School for Advanced Studies, Lucca, Italy, 2IMAGO7 Research Center, Pisa, Italy, 3IRCCS Stella Maris, Pisa, Italy, 4Unit of Neuroradiology, AOUP, Pisa, Italy, 5University of Pisa, Pisa, Italy
Synopsis
The aim of this study is to assess the reproducibility of Quantitative Susceptibility Mapping (QSM), which is crucial to enable the application of this technique in clinical follow-up and multi-center studies. Five healthy subjects underwent multiple QSM acquisition sessions using two MRI systems at different field strength (3T and 7T). Both voxel-wise and automated atlas-based ROI analyses proved the goodness of intra-scanner repeatability and inter-scanner reproducibility, the latter being slightly weaker than the former.
Purpose
Quantitative Susceptibility Mapping (QSM)1–4 provides a way of measuring iron concentration5–7 and myelination8,9 non-invasively, potentially making it an important tool for the study of a host of different pathologies, e.g. neurodegenerative diseases. However, several experimental factors and the physical properties of susceptibility itself, like χ anisotropy, may impair its reliability. To use QSM as a quantitative technique in multi-center studies and for longitudinal follow-ups, it is critical to assess its reproducibility for repeated acquisitions and different field strength. To this purpose, recent studies have been carried out for field strength up to 3T10–12. The aim of this work is to evaluate intra-scanner repeatability and inter-scanner reproducibility on healthy subjects using high (3T) and ultra-high (7T) field MRI systems.Methods
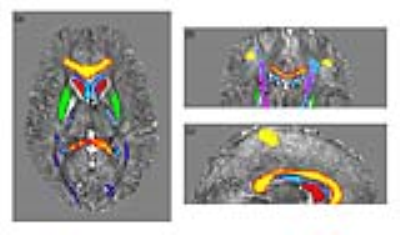
Each of five healthy subjects S1~S5 (29±5 years old; two females) underwent four QSM examinations: two at 3T and two at 7T, using MRI scanners from the same vendor (GE-MR750 and GE-MR950-7T, respectively). Images were acquired by using a 3D Gradient-Recalled Multi-Echo sequence with the following parameters: TE1:ΔTE:TE16=13:3.4:64.4ms, TR=68.1ms and spatial resolution=1×1×1mm3 with whole-brain 3D coverage at 3T; TE1:ΔTE:TE7=5.6:6.4:43.9ms, TR=54.1ms and spatial resolution=0.5×0.5×1mm3 at 7T, covering only the superior part of the cerebrum. The processing pipeline is extensively described in the literature13–16. The last dataset of each subject was acquired no later than one year after the first one. A 3D IR-prepared FSPGR acquisition was acquired to produce T1-weighted volumes for anatomical reference. Several ROIs were selected from probabilistic atlases in FSL (caudate nuclei, putamen and ventricles from Harvard-Oxford Subcortical Structural Atlas17; optic radiation, corpus callosum, corticospinal tract and primary motor cortex from Juelich Histological Atlas18). The ROI masks included the voxels with a probability higher than 65%. The T2*-weighted images were registered to the T1-weighted image by using FSL-FLIRT19, which was then registered to the Montreal Neurological Institute (MNI)20 coordinates via FSL-FLIRT followed by FSL-FNIRT19. Finally, the inverse transformations were applied to the ROIs to work in the original QSM space (Figure 1). For each subject, mean±standard deviation of χ values in each ROI were computed and referenced to the average susceptibility in the bilateral ROI on the ventricles12,21. Intra-scanner repeatability and inter-scanner reproducibility were assessed via both voxel-wise analysis, including voxels from the whole image, and ROI analysis. Orthogonal linear fit and Pearson’s correlation were computed. The variability in each ROI was defined and calculated as the root mean square (RMS) of the difference between the values in two different scans, averaged over subjects.Results
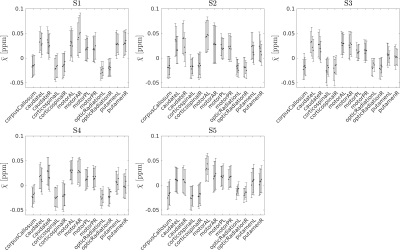
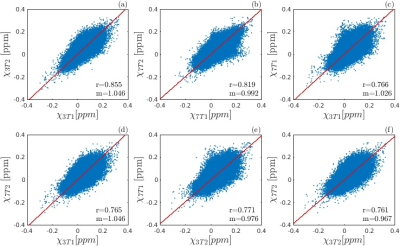
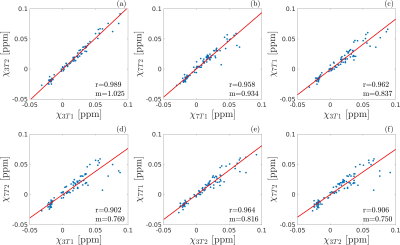
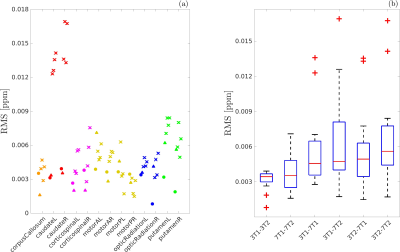
The susceptibility values obtained for each subject in each ROI and each scan are shown in Figure 2. The differences between the mean χ values obtained in the four scans were always less than one standard deviation of the χ distribution within the ROI. The angular coefficient m computed for voxel-wise analysis in Figure 3 differed from 1 by less than 5% for all the plots but the Pearson’s r was lower for inter-scanner correlation (r<0.78) than for intra-scanner correlation (r>0.81). The ROI-based analysis (Figure 4) reported Pearson’s r>0.9, m~1 (difference<7%) for intra-scanner correlation and m<0.84 for inter-scanner reproducibility. The estimated variability RMS for each ROI (Figure 5a), was lower than 0.0071ppm for intra-scanner measurements and 0.0084ppm for inter-scanner data, similarly to what has previously been reported in the literature11. In Figure 5b, the boxplots show the presence of outliers with variability 0.017ppm in inter-scanner reproducibility, corresponding to the caudate nuclei.Discussion
Both 7T and 3T QSM provide data with high repeatability. Intra-scanner reproducibility appears to be slightly weaker especially for ROI-based analysis. The lowest intra-scanner reproducibility is observed for putamen and especially caudate nuclei, despite the good intra-scanner agreement. This result may be due to a confounding effect introduced by the different 3D coverage in 3T and 7T acquisition: in the 7T prescription, which was originally devised to study the motor cortex6, these ROIs are close to the inferior border of the imaging slab and it is likely that the filtering steps in the QSM pre-processing altered their values. It is actually crucial that the target ROIs are fully contained in the imaged volume and far from the borders to achieve high repeatability and reproducibility.Conclusion
This study suggests that QSM at both 3T and 7T shows excellent intra-scanner repeatability and inter-scanner reproducibility with an average precision level of 0.0035ppm and 0.0062ppm respectively. Even though the inclusion of a greater number of subjects is necessary to assess the statistical significance of these results, QSM seems suitable in longitudinal and cross-site studies also at ultra-high field strength.Acknowledgements
No acknowledgement found.References
1. de Rochefort L, Liu T, Kressler B, Liu J, Spincemaille P, Lebon V, Wu J, Wang Y. Quantitative susceptibility map reconstruction from MR phase data using bayesian regularization: Validation and application to brain imaging. Magn Reson Med. 2010;63(1):194-206.
2. Wang Y, Liu T. Quantitative susceptibility mapping (QSM): Decoding MRI data for a tissue magnetic biomarker. Magn Reson Med. 2015;73(1):82-101.
3. Haacke EM, Liu S, Buch S, Zheng W, Wu D, Ye Y. Quantitative susceptibility mapping: current status and future directions. Magn Reson Imaging. 2015;33(1):1-25.
4. Deistung A, Schweser F, Reichenbach JR. Overview of quantitative susceptibility mapping. NMR Biomed. 2016.
5. Barbosa JHO, Santos AC, Tumas V, Liu M, Zheng W, Haacke EM, Salmon CEG. Quantifying brain iron deposition in patients with Parkinson’s disease using quantitative susceptibility mapping, R2 and R2*. Magn Reson Imaging. 2015;33(5):559-565.
6. Costagli M, Donatelli G, Biagi L, Caldarazzo Ienco E, Siciliano G, Tosetti M, Cosottini M. Magnetic susceptibility in the deep layers of the primary motor cortex in Amyotrophic Lateral Sclerosis. NeuroImage Clin. 2016;12:965-969.
7. Acosta-Cabronero J, Cardenas-Blanco A, Betts MJ, Butryn M, Valdes-Herrera JP, Galazky I, Nestor PJ. The whole-brain pattern of magnetic susceptibility perturbations in Parkinson’s disease. Brain. 2017;140(1):118-131.
8. Liu C, Li W, Johnson GA, Wu B. High-field (9.4T) MRI of brain dysmyelination by quantitative mapping of magnetic susceptibility. Neuroimage. 2011;56(3):930-938.
9. Argyridis I, Li W, Johnson GA, Liu C. Quantitative magnetic susceptibility of the developing mouse brain reveals microstructural changes in the white matter. Neuroimage. 2013;88C:134-142.
10. Deh K, Nguyen TD, Eskreis-Winkler S, Prince MR, Spincemaille P, Gauthier S, Kovanlikaya I, Zhang Y, Wang Y. Reproducibility of quantitative susceptibility mapping in the brain at two field strengths from two vendors. J Magn Reson Imaging. 2015;42(6):1592-1600.
11. Lin P-Y, Chao T-C, Wu M-L. Quantitative susceptibility mapping of human brain at 3T: a multisite reproducibility study. AJNR Am J Neuroradiol. 2015;36(3):467-74.
12. Feng X, Deistung A, Reichenbach JR. Quantitative susceptibility mapping (QSM) and R2* in the human brain at 3 T: Evaluation of intra-scanner repeatability. Z Med Phys. 2017.
13. Lancione M, Tosetti M, Donatelli G, Cosottini M, Costagli M. The impact of white matter fiber orientation in single-acquisition quantitative susceptibility mapping. NMR Biomed. 2017;30(11).
14. Li W, Wu B, Liu C. Quantitative susceptibility mapping of human brain reflects spatial variation in tissue composition. Neuroimage. 2011;55(4):1645-1656.
15. Schweser F, Deistung A, Lehr BW, Reichenbach JR. Quantitative imaging of intrinsic magnetic tissue properties using MRI signal phase: an approach to in vivo brain iron metabolism? Neuroimage. 2011;54(4):2789-2807.
16. Li W, Wang N, Yu F, Han H, Cao W, Romero R, Tantiwongkosi B, Duong TQ, Liu C. A method for estimating and removing streaking artifacts in quantitative susceptibility mapping. Neuroimage. 2015;108:111-122.
17. Frazier JA, Chiu S, Breeze JL, Makris N, Lange N, Kennedy DN, Herbert MR, Bent EK, Koneru VK, Dieterich ME, Hodge SM, Rauch SL, Grant PE, Cohen BM, Seidman LJ, Caviness VS, Biederman J. Structural Brain Magnetic Resonance Imaging of Limbic and Thalamic Volumes in Pediatric Bipolar Disorder. Am J Psychiatry. 2005;162(7):1256-1265.
18. Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K, Zilles K. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. 2005.
19. Jenkinson M, Beckmann CF, Behrens TEJ, Woolrich MW, Smith SM. FSL. Neuroimage. 2012;62:782-790.
20. Mazziotta JC, Toga AW, Evans A, Fox P, Lancaster J. A probabilistic atlas of the human brain: theory and rationale for its development. The International Consortium for Brain Mapping (ICBM). Neuroimage. 1995;2(2):89-101.
21. Straub S, Schneider TM, Emmerich J, Freitag MT, Ziener CH, Schlemmer H-P, Ladd ME, Laun FB. Suitable reference tissues for quantitative susceptibility mapping of the brain. Magn Reson Med. 2017;78(1):204-214.
Figures