2217
QSM-MRI reveals increased brain iron deposition in anemia patients with blood transfusion1Biomedical Engineering, University of Southern California, Los Angeles, CA, United States, 2Neuroscience Graduate Program, University of Southern California, Los Angeles, CA, United States, 3Ming Hsieh Department of Electrical Engineering, University of Southern California, Los Angeles, CA, United States, 4Cardiology, Children's Hospital Los Angeles, Los Angeles, CA, United States
Synopsis
Sickle cell patients identified with high stroke risks and other genetically anemic patients are treated with chronic blood transfusions. Unfortunately, transfusions may cause iron overload. While transfusion-related iron overload has been shown in other major organs, less has been explored whether it impacts brain. This study compares brain iron content measured by quantitative susceptibility mapping (QSM) in 17 healthy controls and 33 patients with sickle cell or other types of anemia. We found significantly higher iron in the putamen of anemia patients receiving blood transfusion. The result of this study can provide insights on the neurological effects of blood transfusions.
Introduction
Blood transfusion therapy is used to treat patients with chronic anemia and hemoglobinopathy. Patients with sickle cell disease (SCD) undergo monthly transfusion therapy if identified with high stroke risks and thalassemia patients require the treatment to compensate for low hemoglobin levels. Unfortunately, transfusions expose patients to abnormally high levels of iron that deposit into tissue that may cause iron overload and oxidative tissue damage. While iron overload has been shown in other major organs of the body caused by chronic transfusion, less has been explored whether it impacts brain. We aim to compare brain iron content measured by quantitative susceptibility mapping (QSM) in transfusion patients, with and without SCD, and healthy controls.Methods
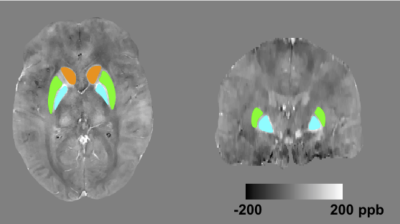
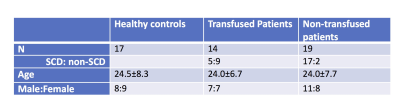
Cohort: Data from 22 SCD patients, 11 non-sickle anemia patients and 17 healthy controls were acquired (Table 1). All subjects provided written consent to a protocol approved by the Committee on Clinical Investigation (CCI#11-00083). Data acquisition: Images were acquired on a clinical 3 T Philips system with a 32-channel RF coil. The 3D gradient echo sequence had parameters: TR = 30 ms, α = 25°, 4 echoes: TE1 = 4.94 ms, ΔTE = 5.2 ms, FOV = 210 x 190 x 120 mm, spatial resolution: 0.6 x 0.6 x 1.3 mm3, SENSE acceleration rate = 2 in the phase-encoding direction, BW = 289 Hz/pix and total acquisition time = 6 mins 50 s. T1-W images were acquired using a 3D FLASH sequence to allow semi-automated region-of-interest (ROI) segmentation. ROI identification: Figure 1 shows the ROIs investigated, which include bilateral caudate nucleus (CN), putamen (PT) and globus pallidus(GP). ROI segmentation of CN, PT and GP was performed on T1-W images by atlas registration and then manually edited by an expert. The labelled ROIs were then registered onto QSM coordinates. Susceptibility quantification: For each subject, phase images were fitted to generate a B0 field map. Brain extraction and phase unwrapping was performed using FSL. Background field was removed using PDF1. Unreliable phase voxels were identified and removed from the brain mask for subsequent processing2. L1-regularized field-to-susceptibility inversion was performed to derive the susceptibility map (lambda = 4x10-4)3. Intra-ROI average susceptibility were computed. Multi-echo magnitude images from the QSM data were fitted to generate R2* maps. Intra-ROI average R2* were computed. Analysis: Step-wise multivariate regression analysis was performed on the total population. Independent variables include age, gender, hemoglobin (g/dL) and transfusion state. Variables were only retained in the final model for p < 0.05.Result
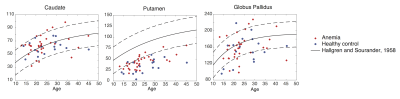
Figure 2 compares susceptibility measurement in our study with an empirical formula derived by Hallgren and Sourander in a post-mortem study4. Iron content (in g/100g fresh weight) measured using staining in their study was converted into susceptibility values based on Ref 5. QSM-based measurement in the caudate and globus pallidus matches with Hallgren and Sourander’s study, whereas measurement in the putamen was lower in our study.
Figure 3 summaries the step-wise multivariate regression analysis. Both susceptibility (Figure 3a) and R2* (Figure 3b) measurements show statistical significance of 1) positive dependence on age in PT and GP and 2) dependence on transfusion in PT. Significant age dependence in CN was only shown in susceptibility measurement.
Remarkably high susceptibility of the putamen (Figure 4a, red arrow) was observed in transfused patients. Figure 4b shows that iron in the putamen varies linearly with log-transformed age. Transfused patients exhibited susceptibility when tested against non-transfused patients grouped with healthy controls (controlled for age; Figure 4c). No difference was found between non-transfused patients and healthy controls.
Discussion
In this study, we compared magnetic susceptibility in the basal ganglia nuclei in transfusion patients, with and without SCD, and healthy controls. Patients and controls demonstrated expected iron accumulation with age, in good agreement with autopsy and previous QSM analyses. While our putamen iron estimates are lower compared with Hallgren and Sourander’s study, they are comparable with prior QSM findings, suggesting that the differences may arise from changes in putamen water content between in-vivo and post-mortem measurements.
Chronically transfused patients exhibited higher iron content (by susceptibility and R2* metrics) in the putamen but not the other brain nuclei..It is unclear whether this iron results as an extension of somatic iron overload or other aspects of chronic transfusion therapy (such as immune modulation). We are investigating the functional correlates of increased putamen iron in these patients as well as additional potential predictors.
Acknowledgements
This work is supported by the National Heart Lung and Blood Institute (1U01HL117718-01).References
1. Liu T, Khalidov I, de Rochefort L, et al. A novel background field removal method for MRI using projection onto dipole fields (PDF). NMR Biomed. 2011;24(9):1129-1136.
2. Bilgic B, Pfefferbaum A, Rohlfing T, Sullivan E V., Adalsteinsson E. MRI estimates of brain iron concentration in normal aging using quantitative susceptibility mapping. Neuroimage. 2012;59(3):2625-2635.
3. Schweser F, Deistung A, Lehr BW, Reichenbach JR. Quantitative imaging of intrinsic magnetic tissue properties using MRI signal phase: an approach to in vivo brain iron metabolism?. Neuroimage. 2011 Feb 14;54(4):2789-807.
4. Hallgren B, Sourander P. THE EFFECT OF AGE ON THE NON-HAEMIN IRON IN THE HUMAN BRAIN. J Neurochem. 1958;3(1):41-51.
5. Langkammer C, Schweser F, Krebs N, Deistung A, Goessler W, Scheurer E, Sommer K, Reishofer G, Yen K, Fazekas F, Ropele S. Quantitative susceptibility mapping (QSM) as a means to measure brain iron? A post mortem validation study. Neuroimage. 2012 Sep 30;62(3):1593-9.
Figures