2209
BuckyBall: Reproducible gradient-echo MRI measurements with variable magnetic field directions1Centre for Medical Image Computing, Department of Computer Science, University College London, London, United Kingdom, 2Institute of Imaging Science, Department of Biomedical Engineering, Vanderbilt University, Nashville, TN, United States
Synopsis
The direction of the external magnetic field is typically fixed, although it is well-known that the signal of various MR modalities in brain white matter depends on the magnetic field direction. This work presents a general framework for analysing B0-direction dependent contrast. Specifically, we have developed a holder device, called BuckyBall, that enables the uniform orientation of the scanned object in a reproducible manner. Its feasibility and practicality are demonstrated in a multi-echo gradient-echo experiment with 50 unique magnetic field directions using a monkey brain sample.
Introduction
The magnitude and phase of the gradient-echo signal in brain white matter does not only depend on microscopic tissue features, but also on the orientation of the nerve fibres relative to the external magnetic field1–3. The B0-direction dependence potentially impact the interpretation of gradient-echo measurements, as it is not straightforward to differentiate between directional tissue heterogeneity and microscopic susceptibility effects, where the latter are mainly attributed to myelin microstructure4–6. Consequently, there is a pressing need to study the MR signal in dependence on the direction of the magnetic field, with the aim of disentangling multiple signal contributors and hence gaining deeper insight into the underlying mechanisms of signal formation.Methods
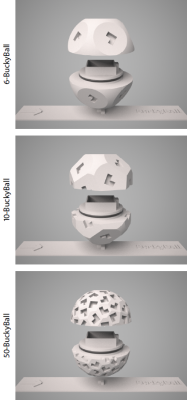
BuckyBall. To measure at multiple directions of the external magnetic field, we developed a holder device, which we refer to as BuckyBall, that allows us to rotate the sample reproducibly. The objective is to arrange n magnetic field directions B0(i) for i = 1, ..., n, together with the opposite directions –B0(i), as evenly as possible. The faces of the BuckyBall, which are endowed with L-shape encodings, are designed in such a way that the rotations of the sample holder give rise to the specified set of magnetic field directions. The face encoding is crucial as otherwise the BuckyBall, when placed on the table, would be freely rotatable around its axis, thus compromising the replicability of the experiment. Figure 1 depicts the BuckyBall for 6, 10 and 50 magnetic field directions uniformly distributed over a hemisphere. The 50-BuckyBall was manufactured using 3D printing with a 3D Systems ProJet 3500 HDMax system at ultra-high resolution.
Materials & Experiments. We conducted an ex-vivo study with a brain sample from a female adult owl monkey, which was perfusion fixed and placed in PFA with 10% sucrose until washing with PBS before scanning. The sample was measured in the 50-BuckyBall holder (Figure 1) filled with Fomblin on a 15.2T Bruker BioSpec scanner. A 3D gradient-echo experiment with 16 echoes (flip angle 45°, TE1 = 1.837 ms, ESP = 3 ms, TR = 100 ms) was performed at 50 magnetic field directions. In addition, we ran a 3D diffusion FSE scan with 60 unique gradient directions evenly distributed over two b-shells of 3000 and 6000 s/mm2. The image resolution of all scans was 250 µm isotropic.
Data processing. The gradient-echo and diffusion images were first co-registered. After phase unwrapping7, we calculated the frequency difference signal8,9, which eliminated time-independent frequency shifts and non-local susceptibility effects, and then subtracted the background field using a 3D fourth-order polynomial. To study the orientation dependence of the gradient-echo MR signal, we have developed a unifying rank-2 tensor framework for modelling and estimating the B0-direction anisotropy for various signal features, thereby extending previous work10,11 that assumed rotational symmetry. Specifically, we demonstrate the quantitative recovery of the full R2*-relaxivity and gradient-echo frequency tensors. Following diffusion data preprocessing, we estimated the diffusion tensor12 and subsequently compared the directional information retrieved from the various MR modalities.
Results
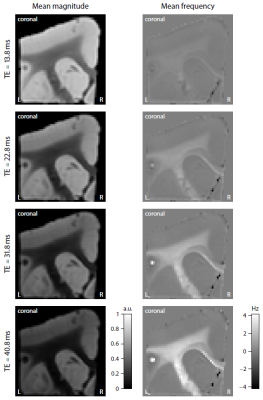
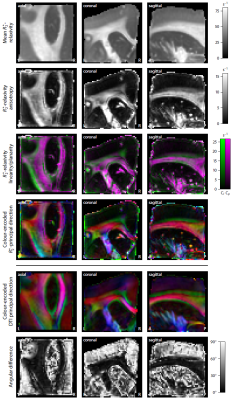
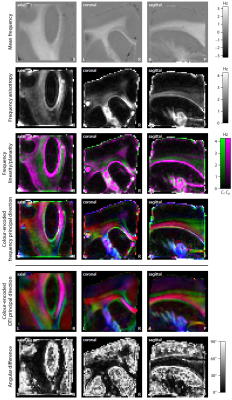
Figure 2 depicts the magnitude and frequency of the mean gradient-echo signal, which was calculated over the magnetic field directions. An interesting result is that the mean frequency in white matter varies with echo time, noting that the signal frequency was referenced to the mean value in grey matter. Figures 3 and 4 show the estimation of the full R2*-relaxivity and frequency tensor obtained from the magnitude and phase signal, respectively. The first row maps the mean eigenvalue of the tensor, while the second row displays the tensor anisotropy, which is defined as the absolute difference between the axial and radial eigenvalues. We observe both high R2*-relaxivity and gradient-echo frequency directionality in white matter. The third row maps the geometrical shape of the tensor13. Green indicates a linear geometry, magenta a planar shape, and black an isotropic tensor, which can be mainly found in grey matter. The fourth row shows the colour-encoded principal direction, which is compared to the directional information retrieved from diffusion tensor imaging (DTI). The angular difference is typically low in white matter regions, whereas the high error measures in grey matter are due to the low myelin content deteriorating the directional sensitivity.Discussion
In this work we have introduced the BuckyBall holder specifically designed to study the directional dependence of the MR signal on the external magnetic field, here demonstrated in gradient-echo measurements. The holder device is simple to set up, easy to use and highly adjustable to experimental needs. Indeed, we have achieved the highest angular resolution and most uniform coverage of magnetic field directions thus far. The developed BuckyBall holder and unifying tensor framework facilitate verifiable research on B0-direction dependent contrast in orientationally heterogeneous material.Acknowledgements
The authors thank the Jon Kaas lab and Kurt Schilling (both Vanderbilt University) for providing the brain sample. This work was supported by grants UK EPSRC EP/M020533/1, EPSRC EP/N018702/1, EU H2020 634541-2 and US NIH/NIBIB EB019980. The software is available online at https://ekaden.github.io.References
1. He X and Yablonskiy DA. Biophysical mechanisms of phase contrast in gradient echo MRI. Proceedings of the National Academy of Sciences of the United States of America, 106:13558–13563, 2009.
2. Bender B and Klose U. The in vivo influence of white matter fiber orientation towards B0 on T2* in the human brain. NMR in Biomedicine, 23:1071–1076, 2010.
3. Lee J, Shmueli K, Fukunaga M, van Gelderen P, Merkle H, Silva AC and Duyn JH. Sensitivity of MRI resonance frequency to the orientation of brain tissue microstructure. Proceedings of the National Academy of Sciences of the United States of America, 107:5130–5135, 2010.
4. Liu C, Li W, Johnson GA and Wu B. High-field (9.4 T) MRI of brain dysmyelination by quantitative mapping of magnetic susceptibility. NeuroImage, 56:930–938, 2011.
5. Lee J, Shmueli K, Kang B-T, Yao B, Fukunaga M, van Gelderen P, Palumbo S, Bosetti F, Silva AC and Duyn JH. The contribution of myelin to magnetic susceptibility-weighted contrasts in high-field MRI of the brain. NeuroImage, 59:3967–3975, 2012.
6. Lodygensky GA, Marques JP, Maddage R, Perroud E, Sizonenko SV, Hüppi PS and Gruetter R. In vivo assessment of myelination by phase imaging at high magnetic field. NeuroImage, 59:1979–1987, 2012.
7. Bioucas-Dias JM and Valadão G. Phase unwrapping via graph cuts. IEEE Transactions on Image Processing, 16:698–709, 2007.
8. Schweser F, Deistung A, Güllmar D, Atterbury M, Lehr BW, Sommer K and Reichenbach JR. Non-linear evolution of GRE phase as a means to investigate tissue microstructure. In Proceedings of the 19th Annual Meeting of the ISMRM, page 4527, 2011.
9. Wharton S and Bowtell R. Fiber orientation-dependent white matter contrast in gradient echo MRI. Proceedings of the National Academy of Sciences of the United States of America, 109:18559–18564, 2012.
10. Wharton S and Bowtell R. Gradient echo based fiber orientation mapping using R2* and frequency difference measurements. NeuroImage, 83:1011–1023, 2013.
11. Aggarwal M, Kageyama Y, Li X and van Zijl PC. B0-orientation dependent magnetic susceptibility-induced white matter contrast in the human brainstem at 11.7T. Magnetic Resonance in Medicine, 75:2455–2463, 2016.
12. Basser PJ, Mattiello J and Le Bihan D. Estimation of the effective self-diffusion tensor from the NMR spin echo. Journal of Magnetic Resonance, Series B, 103:247–254, 1994.
13. Westin C-F, Maier SE, Mamata H, Nabavi A, Jolesz FA and Kikinis R. Processing and visualization for diffusion tensor MRI. Medical Image Analysis, 6:93–108, 2002.
Figures