2188
Systematic Assessment of Multi-Echo Dynamic Susceptibility Contrast (DSC) MRI using a Digital Reference Object (DRO)1Translational Bioimaging Group, Barrow Neurological Institute, Phoenix, AZ, United States
Synopsis
Brain tumor dynamic susceptibility contrast (DSC) MRI is adversely impacted by contrast agent leakage that results in confounding T1 and T2* effects. While multi-echo acquisitions remove T1 leakage effects, there is no consensus on the optimal set of acquisition parameters. Using a validated DSC-MRI digital reference object (DRO), we assessed the influence of preload dosing, pulse sequence parameters (number of echoes, TEs, TR, FA), and leakage correction method on cerebral blood volume (CBV) accuracy. This computational approach permits the systematic evaluation of a wide range of acquisition strategies to determine the optimal multi-echo DSC-MRI perfusion protocol.
Introduction
Relative cerebral blood volume (rCBV) measures obtained from DSC-MRI are widely used in the diagnosis and treatment of brain tumors [1,2]. However, contrast agent leakage effects can limit the reliability of rCBV measurements [1,3]. Both acquisition (contrast agent dosing and pulse sequence parameters) and post-processing methods have been proposed to minimize or remove these effects. By acquiring multiple echoes [4], T1 leakage effects can be removed and may provide more robust rCBV measures [5]. Unfortunately, there is no consensus on the optimal set of acquisition parameters for multi-echo DSC-MRI, and experimentally assessing these methods is challenging and impractical. The purpose of this study is to leverage a digital reference object (DRO) to systematically evaluate a wide range of acquisition strategies to determine the optimal multi-echo DSC-MRI perfusion protocol.Methods
The details of the population-based DRO were published previously [6]. Briefly, the DRO calculates the MR signal from realistic 3D tissue structures, with the cells and vessels simulated as ellipsoids packed around randomly oriented cylinders. To ensure clinical relevance, the DRO was trained and validated using two distinct datasets from glioblastoma patients. For this study, the DRO MRI data were simulated at 3T. To test the optimal combination of echo times, four echo times were simulated (20, 30, 40, and 50 ms). Each echo time was assessed individually (single-echo); dual-echo combinations were 20 and 30 ms, 20 and 50 ms, and 40 and 50 ms. A multi-echo (ME) fit to all 4 echoes was also assessed. The MRI protocol also included three flip angles (30, 60, and 90°) and three repetition times (TRs, 1, 1.5, and 2 s). Two injection protocols were simulated: no preload with single-dose bolus (0+1) and single-dose preload and bolus (1+1). For each parameter combination, the DRO produced 10,000 voxels with varying CA leakage effects. A parallel set of 10,000 voxels was simulated without leakage (Ktrans = 0) for each parameter combination. Leakage correction was performed using the standard Boxerman-Schmainda-Weisskoff (BSW) method [7,8]. An accuracy and precision (AP) index, defined as the concordance correlation coefficient (CCC) – |coefficient of variation (CV)|, was used to determine the best possible parameter combinations.Results / Discussion
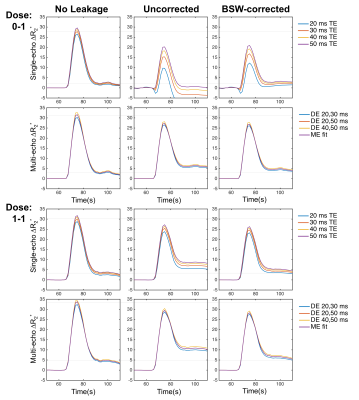
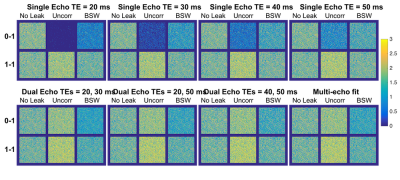
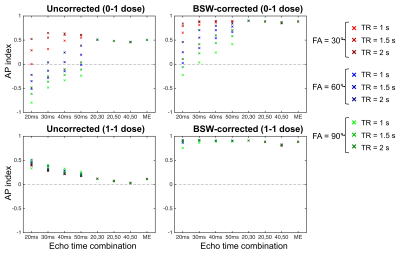
Figure 1 shows the single-echo and multi-echo ΔR2* curves with and without preload and leakage correction (FA = 60°, TR = 1.5s). Although preload reduces T1 leakage effects for the single-echo combinations, it further emphasizes T2* leakage effects for the multi-echo combinations, as T1 leakage effects are inherently removed. The multi-echo ΔR2* is highly consistent across all echo combinations for both dosing combinations. Compared to the no leakage ΔR2*, both the uncorrected and BSW-corrected peak ΔR2* are reduced due to CA leakage, with the BSW correction having the highest impact after the peak. The DRO rCBV maps are shown in Figure 2 for all echo time combinations with and without preload and leakage correction (FA = 60°, TR = 1.5s). T1 leakage effects lead to underestimated rCBV for single-echo without preload, while T2* leakage effects cause overestimation for single-echo with preload and all multi-echo combinations with and without preload. Leakage correction improves rCBV accuracy and appears to adequately correct T2* leakage effects. With multi-echo acquisitions, the TE combination has minimal impact on rCBV, and multi-echo acquisitions may obviate the need for preload dosing. The AP index was calculated for all parameter combinations (TR, FA, and TE). Multi-echo is more consistent and closer to 1 (optimal) than the single-echo options, with minimal benefit from use of a preload. For multi-echo, the choice of TR and FA have little impact on the AP index, indicating the potential for significant flexibility in acquisition parameters. Inclusion of a shorter multi-echo TE slightly improves AP index. BSW correction improves the AP index in all cases. Work is ongoing to expand the DRO parameter space to a wider range of TEs, including shorter TEs.Conclusions
Contrast agent extravasation reduces the reliability of DSC-MRI brain tumor perfusion measures. Using a DRO, we have systematically assessed four multi-echo combinations across two dosing schemes, three TRs, three FAs, and one leakage correction method. Overall, the multi-echo acquisitions were more robust than single-echo acquisitions. The dual-echo combinations performed as well as the multi-echo fit, indicating that additional echoes (>2) may not be necessary. The use of multi-echo acquisitions provides significant pulse sequence flexibility, essentially decoupling both TR and FA from rCBV accuracy. The DRO also demonstrates that multi-echo acquisitions do not benefit from a preload injection. Finally, leakage correction was found to improve rCBV accuracy in all cases.Acknowledgements
This work was supported by the Arizona Biomedical Research Commission (ADHS16-162414) and NIH/NCI 2R01CA158079.References
[1] Boxerman JL, Schmainda KM,
Weisskoff RM. Relative cerebral blood volume maps corrected for contrast agent
extravasation significantly correlate with glioma tumor grade, whereas
uncorrected maps do not. AJNR Am J Neuroradiol 2006;27:859–67.
[2] Schmainda KM, Prah M, Connelly
J, Rand SD, Hoffman RG, Mueller W, et al. Dynamic-susceptibility contrast agent
MRI measures of relative cerebral blood volume predict response to bevacizumab
in recurrent high-grade glioma. Neuro Oncol 2014;16:880–8.
doi:10.1093/neuonc/not216.
[3] Stokes AM, Semmineh N,
Quarles CC. Validation of a T1 and T2* leakage correction method based on
multiecho dynamic susceptibility contrast MRI using MION as a reference
standard. Magn Reson Med 2016;76:613–25. doi:10.1002/mrm.25906.
[4] Vonken E, van Osch M,
Bakker C, Viergever M. Measurement of cerebral perfusion with dual-echo
multi-slice quantitative dynamic susceptibility contrast MRI. J Magn Reson
Imaging 1999;10:109–17.
[5] Paulson ES, Schmainda KM.
Comparison of Dynamic Susceptibility-weighted Contrast-enhanced MR Methods:
Recommendations for Measuring Relative Cerebral Blood Volume in Brain Tumors.
Radiology 2008;249:601–13. doi:doi:10.1148/radiol.2492071659.
[6] Semmineh NB, Stokes AM,
Bell LC, Boxerman JL, Quarles CC. A Population-Based Digital Reference Object
(DRO) for Optimizing Dynamic Susceptibility Contrast (DSC)-MRI Methods for
Clinical Trials. Tomography 2017;3:41–9. doi:10.18383/j.tom.2016.00286.
[7] Boxerman JL, Schmainda KM,
Weisskoff RM. Relative Cerebral Blood Volume Maps Corrected for Contrast Agent
Extravasation Significantly Correlate with Glioma Tumor Grade, Whereas
Uncorrected Maps Do Not. Am J Neuroradiol 2006;27:859–67.
[8] Weisskoff RM, Boxerman JL, Sorensen AG, Kulke SM,
Campbell TA, Rosen BR. Simultaneous blood volume and permeability mapping using
a single Gd-based contrast injection. Proc. 2nd Annu. Meet. SMRM, San
Francisco, CA, USA: 1994, p. 279.
Figures