2177
Influence of background suppression and retrospective realignment on free-breathing renal perfusion imaging using ASL1Division of MRI Research, department of Radiology, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, MA, United States, 2Global MR Applications and Workflow, GE Healthcare, Boston, MA, United States
Synopsis
While a consensus exists on the benefits of background suppression for brain ASL to reduce physiological noise, conflicting results have been presented for renal applications. Furthermore, bulk motion management remains a challenge for clinical applications. In the current work, we investigate the effects and interactions between background suppression and retrospective motion-correction when used for single-slice free-breathing renal ASL. We emphasize the influence of BS and motion-correction on thermal and physiological noise levels and show that BS is critical for renal ASL using pCASL while retrospective motion-compensation helps in increasing image sharpness.
Introduction
Although used in several clinical research studies (e.g. cancer or chronic kidney disease)1,2, ASL-based renal perfusion measurement is still considered a niche research technique due to several challenges, especially motion (bulk and physiological) corruption. While several approaches have been used to tackle the motion issue3,4,5, no consensus has yet been reached on the use of background suppression (used to reduce static tissue signal in ASL) to increase the robustness of renal ASL. In the current work, we propose a characterization of the influence of BS as well as retrospective motion-correction (MC) strategies on physiological noise and overall image quality in renal ASL.Material and Methods
5 subjects (3M,2F,35±5yo) were scanned at 3T (Discovery MR750, GE Healthcare) with a 32-ch body coil. ASL data were acquired with a pseudo-continuous labeling6 (repetition rate 1.18ms, average B1=1.4μT, Gmax/Gav=3.5/0.5mT/m)7 during 1.5s followed by a 1.5s post-labeling delay. The labeling plane was positioned 80mm above the center of the imaging slice to label descending aortic blood. BS was achieved by a series of saturation and selective (FOCI) and non-selective (Hyperbolic-secant) pulses8. Variable BS schemes were first optimized to reach an assigned background signal level (<2%,<5%,<10% and <20%) for T1 between 250ms and 4s by modeling BS timing with the following equation9,10 within MATLAB for n inversion pulses applied at a time ti after a presaturation pulse applied at time Q (4.1s): $$M_{z}(T_{1})=1+(-1)^{n+1}e^{-\frac{Q}{T_{1}}}+2\sum_{i=1}^n(-1)^{i}e^{-\frac{t_{i}}{T_{1}}}$$ All images were acquired during free breathing using a single-shot FSE readout (single coronal slice, TR/TE=6000/45ms, matrix 1282). 3 additional references images were acquired at the beginning of each scan (PD-w and 2-IR), as well as 14 label/control pairs leading to a total Tacq=3min for each BS scheme.
All data were reconstructed offline. To evaluate the efficiency of retrospective MC with various BS levels, a retrospective MC was implemented using the ANTs libraries11. Each ASL image was masked by the BS spatial extent, then each control/label images was registered to the 1st control/label image acquired using a rigid initialization followed by a non-linear B-Spline-SyN12 registration (cross-correlation cost function, 2 steps registration, 120x60 iterations, gradient step=0.01 and 3rd degree B-Spline interpolation). Finally, all registered data were averaged in image space followed by subtraction and perfusion quantification using a standard kinetic model13. Temporal SNR and SNR were calculated to assess physiological and thermal noise contribution for each BS level with/without motion-correction. Finally, BS/MC influence on quantitative renal blood-flow (RBF) was also evaluated.
Results
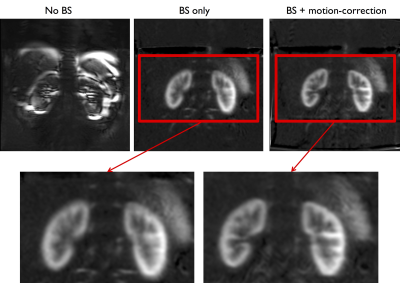
It can be seen on fig.1 that BS appears critical for free-breathing renal pCASL. The MC further increases the sharpness of the perfusion image with better depiction of the renal morphology.
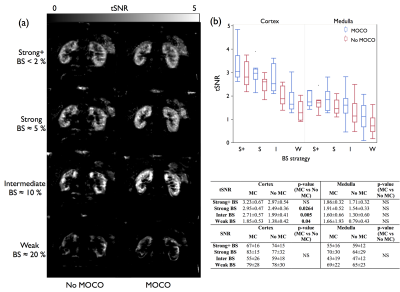
Visual (fig.2a) and quantitative (fig.2b) analysis shows that the heaviest levels of BS provided higher tSNR with/without MC, suggesting a reduction of temporal fluctuations of the ASL signal partially attributed to physiological noise. Furthermore, better tSNR homogeneity is systematically obtained with the highest BS levels, while no SNR differences could be seen. Achieving BS with less inversion pulses should lead in an SNR increase due to higher global inversion efficiency8.
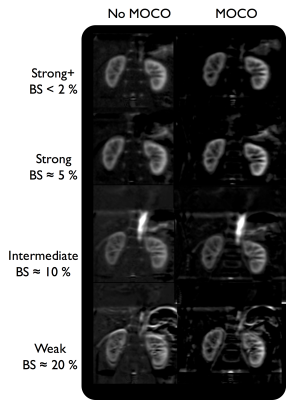
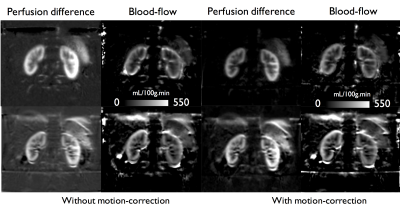
We observed an increase in image quality of perfusion images (fig.3) with the use of MC except for the lowest BS scheme. Similarly, we could see that when the BS signal increases, additional background noise appears due to increased subtraction errors. This affects the quantitative RBF (fig.4), for which although no significant difference could be found due to BS and MC, a reduction by up to 40% of RBF STD was observed in the cortex. For instance, the highest BS level, thanks to the good suppression of static tissues, provides images less dominated by background noise. In addition to that, the MC seems more efficient on heavily BS images compared to an intermediate BS level, leading to a higher cortical/medullary contrast.
Discussion and conclusions
We have shown that BS is highly desirable if not essential for renal perfusion using pCASL with a free-breathing acquisition. Although not as critical as BS, MC is also beneficial in combination with BS by reducing image blurring. Interestingly, it was observed that MC was most efficient when the strongest BS was employed due to the dramatic reduction in background signal. However, extension of the current work to 2D-multislice or 3D readouts14,15 is non-trivial because of loss of BS efficiency across multiple 2D slices and of the segmented nature of 3D readouts. Also, evaluation in clinical cases with renal anatomy alteration or significantly reduced blood-flow should be performed. In conclusion, we demonstrated that BS greatly improves physiological noise levels and allows further image improvement by retrospective MC, facilitating free-breathing renal ASL perfusion measurement.Acknowledgements
No acknowledgement found.References
1. De Bazelaire, C. et al. Arterial spin labeling blood flow magnetic resonance imaging for the characterization of metastatic renal cell carcinoma(1). Acad. Radiol. 12, 347–357 (2005).
2. Mora-Gutiérrez, J. M. et al. Arterial spin labeling MRI is able to detect early hemodynamic changes in diabetic nephropathy. J. Magn. Reson. Imaging JMRI (2017). doi:10.1002/jmri.25717
3. Gardener, A. G. & Francis, S. T. Multislice perfusion of the kidneys using parallel imaging: image acquisition and analysis strategies. Magn. Reson. Med. 63, 1627–1636 (2010).
4. Tan, H., Koktzoglou, I. & Prasad, P. V. Renal perfusion imaging with two-dimensional navigator gated arterial spin labeling. Magn. Reson. Med. 71, 570–579 (2014).
5. Robson, P. M. et al. Strategies for reducing respiratory motion artifacts in renal perfusion imaging with arterial spin labeling. Magn. Reson. Med. 61, 1374–1387 (2009).
6. Dai, W., Garcia, D., de Bazelaire, C. & Alsop, D. C. Continuous flow-driven inversion for arterial spin labeling using pulsed radio frequency and gradient fields. Magn. Reson. Med. 60, 1488–1497 (2008).
7. Zhao, L., Vidorreta, M., Soman, S., Detre, J. A. & Alsop, D. C. Improving the robustness of pseudo-continuous arterial spin labeling to off-resonance and pulsatile flow velocity. Magn. Reson. Med. (2016). doi:10.1002/mrm.26513
8. Garcia, D. M., Duhamel, G. & Alsop, D. C. Efficiency of inversion pulses for background suppressed arterial spin labeling. Magn. Reson. Med. 54, 366–372 (2005).
9. Maleki, N., Dai, W. & Alsop, D. C. Optimization of background suppression for arterial spin labeling perfusion imaging. Magma N. Y. N 25, 127–133 (2012).
10. Mani, S., Pauly, J., Conolly, S., Meyer, C. & Nishimura, D. Background suppression with multiple inversion recovery nulling: applications to projective angiography. Magn. Reson. Med. 37, 898–905 (1997).
11. Avants, B. B. et al. A Reproducible Evaluation of ANTs Similarity Metric Performance in Brain Image Registration. NeuroImage 54, 2033–2044 (2011).
12. Avants, B. B., Epstein, C. L., Grossman, M. & Gee, J. C. Symmetric diffeomorphic image registration with cross-correlation: evaluating automated labeling of elderly and neurodegenerative brain. Med Image Anal 12, 26–41 (2008).
13. Buxton, R. B. et al. A general kinetic model for quantitative perfusion imaging with arterial spin labeling. Magn. Reson. Med. 40, 383–396 (1998).
14. Robson, P. M. et al. Volumetric Arterial Spin-labeled Perfusion Imaging of the Kidneys with a Three-dimensional Fast Spin Echo Acquisition. Acad. Radiol. 23, 144–154 (2016).
15. Cai, Y. et al. Diagnostic value of renal perfusion in patients with chronic kidney disease using 3D arterial spin labeling. J. Magn. Reson. Imaging 46, 589–594 (2017).
Figures