2173
Brain connectivity assessment between rest condition and verbal fluency task through Arterial Spin Labeling1InBrain Lab - FFCLRP, University of Sao Paulo, Ribeirao Preto, Brazil, 2Physics Institute of Sao Carlos, University of Sao Paulo, Sao Carlos, Brazil
Synopsis
Arterial Spin Labeling (ASL) is a method designed to measure blood perfusion. In special, brain perfusion is measured as the cerebral blood flow (CBF), whose time-series fluctuations allow its use in functional analysis. This study aimed to run a dual-echo pseudo-continuous ASL acquisition and analyze its capacity to identify brain networks activated during a verbal fluency task and study the dynamic of brain areas during task and rest conditions. Results showed that it is possible to access language networks based on CBF-ASL, and reported differences in connectivity between both conditions analyzed.
Introduction
Functional magnetic resonance imaging (fMRI) is widely used in the assessment of neurologic disorders1. Initially, specific tasks were drawn and performed to activate the desired brain area and evaluate its functionality. Over the last decade, however, the development and use of resting state (RS) fMRI have changed significantly the approach to study brain functions, having the advantage of no need for the patient to perform tasks during the scanning2. Classically, fMRI explores the blood level oxygen dependent (BOLD) contrast that results from a complex relationship between cerebral blood flow (CBF), cerebral blood volume and cerebral metabolic rate of oxygen. Arterial Spin Labeling (ASL) is a noninvasive MRI-based method to measure perfusion magnetically labeling the arterial blood3. Beyond it noninvasiveness, ASL provides the voxelwise quantification of CBF and the study of brain function and connectivity through fluctuations in CBF time series. Over the last years, interest in functional ASL (fASL) increased, since from a single acquisition quantitative CBF and functional information can be obtained4. Moreover, when compared to BOLD, fASL signal is structurally more specific to neuronal activation because it comes from arterioles. However, ASL has an intrinsic disadvantage of low temporal and spatial resolution. Therefore, the aim of this study was to evaluate the capacity of ASL to identify brain networks activated during a verbal fluency task, and compare their connectivity pattern with the one during resting state.Methods
Healthy adult volunteers (N=9) were scanned in a 3T Philips system equipped with gradients capable of 80mT/m amplitude and 200mT/m/ms slew rate, and a 32-channel head coil. Images were acquired using a dual-echo pseudo-continuous ASL scheme (pCASL) with a GE-EPI sequence (TR/TE1/TE2 = 4000/10/28ms, LD/PLD = 1550/1400ms, FOV = 240x240mm2, matrix = 160x160, 20 6-mm slices). Short TE was used to acquire CBF-based signal, whereas long TE was used for more T2* weighting, resulting in images with BOLD contamination. Two acquisitions were performed: at rest with 48 control/label pairs, and during a semantic verbal fluency (VF) task, with 32 pairs. Images were preprocessed with local scripts in MATLAB and SPM12. Functional connectivity analysis was realized with scripts in MATLAB, R and CONN toolbox. For the connectivity analysis, ten major brain areas related to language processing (table 1) were correlated to all other anatomical areas in both RS and VF conditions. Only significant correlations (p < 0.05) with FDR correction were considered.Results
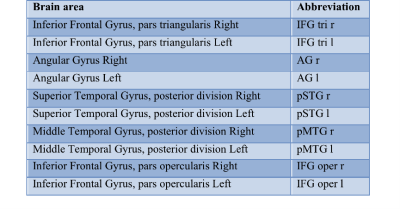
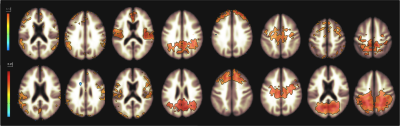
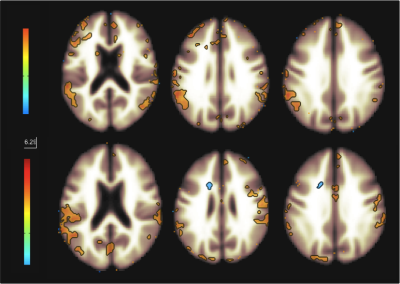
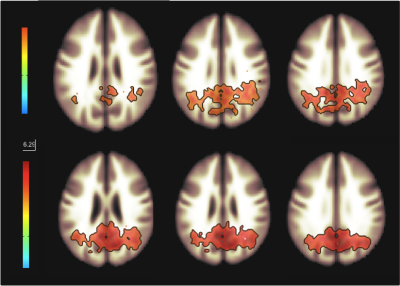
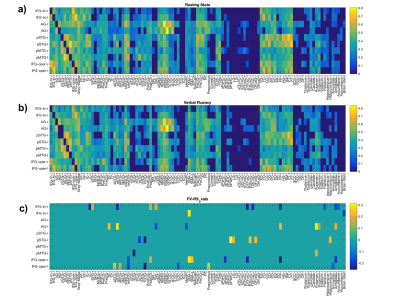
Figure 1 shows functional networks found for VF task (top) and RS (bottom) conditions from CBF time series obtained from the use of short TE. A visual analysis of this figure reveals similar networks. Figures 2 and 3 show, respectively, the language and default mode (DMN) networks for VF (top) and RS (bottom) conditions in which is possible to see a lateralization in language network during task condition and a much strong correlation in DMN for the resting state condition. Figure 4 represents the connectivity matrices for RS (fig.4a) and task conditions (fig.4b) that show almost the same pattern, evidenced by the small differences in correlation between language and attentional areas (fig.4c).Discussion
Despite the results presented in figure 1 look like similar for both conditions, a detailed analysis revealed important differences that characterize each condition. For the task condition, language network showed laterality as previously reported5. In addition, even though the DMN also appeared for the task condition, it showed higher correlations for the RS condition6. Moreover, similar connectivity patterns were observed on the matrices, but with expected differences in correlations between language and attentional areas. The significant values for the difference between RS and VF conditions point to regions related to primary language areas (STG and IFG), attention and memory areas (supplementary motor area, SMA).Conclusion
This study showed the capacity of ASL to identify brain networks during a cognitive brain task through the analysis of CBF fluctuations. It also reported the differences in CBF networks between resting state and a language task condition. Both of these findings might be clinically relevant, since the assessment of language network has several applications, such as tumor and epilepsy. Moreover, since CBF networks are more spatially specific to neural activity, it can provide more precise information to be used clinically. Future analysis of this study might include a comparison of images acquired with both TE values, the increase of the number of subjects and the application in a group of patients.Acknowledgements
CNPq; Capes; FAPESP.References
1. Detre, J.A. and T.F. Floyd, Functional MRI and its applications to the clinical neurosciences. Neuroscientist, 2001. 7(1): p. 64-79.
2. Lee, M.H., et al., Clinical Resting-state fMRI in the Preoperative Setting: Are We Ready for Prime Time? Top Magn Reson Imaging, 2016. 25(1): p. 11-8.
3. Detre, J.A., et al., Perfusion imaging. Magn Reson Med, 1992. 23(1): p. 37-45.
4. Chen, J.J., K. Jann, and D.J. Wang, Characterizing Resting-State Brain Function Using Arterial Spin Labeling. Brain Connect, 2015. 5(9): p. 527-42.
5. Richlan, F., Developmental dyslexia: dysfunction of a left hemisphere reading network. Front Hum Neurosci, 2012. 6: p. 120.
6. Raichle, M.E., et al., A default mode of brain function. Proc Natl Acad Sci U S A, 2001. 98(2): p. 676-82.
Figures