2166
Improved functional Arterial Spin Labeling by spatio-temporal ICTGV denoising1Institute of Medical Engineering, Graz University of Technology, Graz, Austria, 2Institute of Psychology, University of Graz, Graz, Austria, 3Institute of Mathematics and Scientific Computing, University of Graz, Graz, Austria, 4BioTechMed-Graz, Graz, Austria
Synopsis
Functional Arterial Spin Labeling (fASL) provides important information of perfusion changes over time and is therefore suitable for detecting neuronal activation due to cognitive functions or motor tasks. However, the low signal to noise ratio of ASL images restrains its application in clinical and research areas. In this study we propose a method for denoising fASL data using infimal convolution of total generalized variations (ICTGV). Compared to standard Gaussian denoising ICTGV denoising incorporates spatial and temporal information of the perfusion weighted time series. This leads to a substantial improvement in noise-suppression for fASL data.
Introduction
Functional Arterial Spin Labeling (fASL) provides important information of perfusion changes over time due to its sensitivity to blood flow changes.1,2 It is therefore suitable for detecting neuronal activation due to cognitive functions or motor tasks. Compared to blood oxygen level dependent (BOLD) fMRI it has some important advantages: increased spatial accuracy and reproducibility3 and direct cerebral blood flow (CBF) quantification.1,2 However fASL suffers from a low signal-to-noise ratio which restrains its application in clinical and research areas. We propose a method for denoising fASL data using infimal convolution of total generalized variations4 (ICTGV). Compared to standard Gaussian denoising ICTGV incorporates spatial and temporal information for denoising of the perfusion weighted (PW) time series. We evaluated our denoising approach on synthetic and in-vivo data using finger tapping experiments.Methods
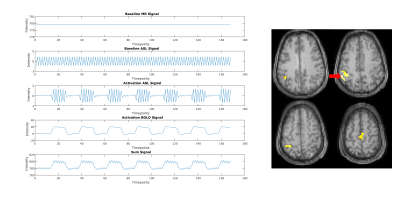
Four healthy volunteers were scanned at a 3T MR system (Magnetom Skyra, Siemens Healthcare, Germany). Pulsed ASL measurements were performed using PICOR-Q2TIPS5 single shot EPI with a 20-channel head coil: 15 slices with 6mm thickness, distance factor 25%, 3x3mm in-plane resolution, 6/8 partial-Fourier, TR/TE = 2500/13ms, TI1/TI2 = 800/1800ms and labeling slice thickness 10cm. A block design paradigm with seven interleaved 30-second periods of rest and finger tapping (right hand) was conducted. Additionally a T1-weighted image was acquired for each subject using a 3D MPRAGE sequence with 1mm isotropic resolution. From one subject a synthetic CBF map was generated based on the T1w image.6 In this synthetic CBF map, voxels corresponding to activations due to motor tasks (voxels in the primary sensorimotor area, supplementary motor area and parietal and parietal associative area) are superimposed with a BOLD effect and ASL activation signal change (30s on/off, 7 runs) as shown in figure 1 and described in7. Zero mean Gaussian noise was added to this synthetic CBF map. ASL data processing and statistical analysis were conducted using MATLAB (Mathworks, Natick, Massachusetts), SPM12, ASL-toolbox8,9 and in-house MATLAB scripts. Prior to denoising the fASL images were motion-corrected, detrended and surround subtraction was applied to eliminate BOLD signal contamination.10 Spatio-temporal denoising of the fASL data is done by solving the following minimization problem using Primal Dual algorithm11:
$$\min_{dM,v} \frac{\lambda}{2}\left\|dM - dM^{\delta}\right\|_2^2 + \gamma_1(s)TGV_{\alpha1,\alpha0,tsw1}(dM - v) + \gamma_2(s)TGV_{\alpha1,\alpha0,tsw2}(v)$$
where $$$\alpha1\,$$$and$$$\,\alpha0\ $$$ are fixed model parameters4, tsw1 and tsw2 are weightings between spatial and temporal regularization, the parameters s controls the weighting between the two TGV terms and is calculated as described in4, $$$\lambda$$$ denote the regularization parameter for the difference image dM, $$$dM^{\delta}$$$ are the acquired 4D PWI-images and $$$dM$$$ is the 4D denoised PWI image. For comparison the perfusion weighted images were denoised using a conventional 3D-Gaussian kernel of 6mm FWHM. CBF-maps were calculated from the perfusion weighted images using a general kinetic model.12 Task-based fASL analysis was performed in SPM12 for each subject. A general linear model (GLM) was used. The statistical maps were thresholded at p<0.05 after family-wise-error correction on overlayed on the coregistered T1w-images.
Results and Discussion
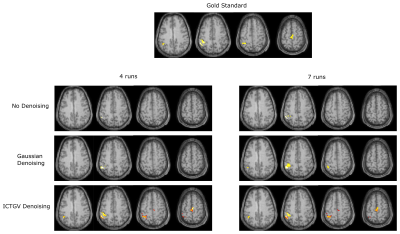
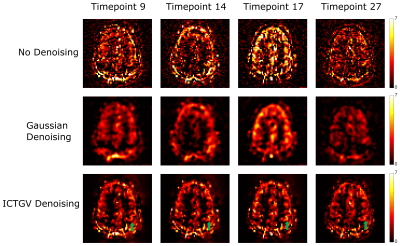
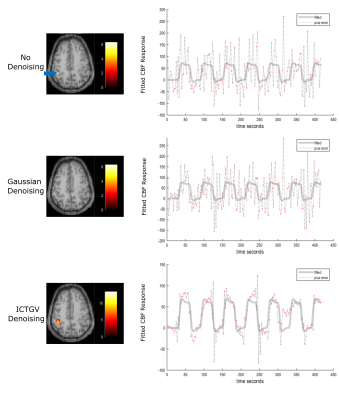
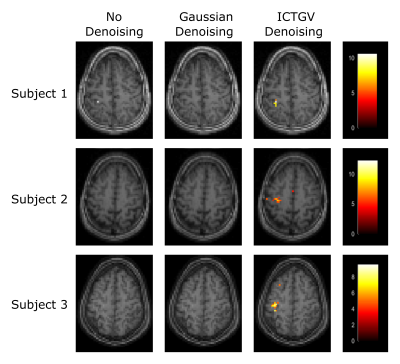
Figure 1 shows the simulated images with activations in areas as previously described and a corresponding time course of one representive voxel (red arrow). Figure 2 shows the activation-maps (FWE, p<0.05) overlayed with a T1w-image using no denoising, Gaussian denoising and the proposed denoising method as preprocessing step. Compared to no denoising and Gaussian denoising the incorporation of temporal information in the proposed denoising method results in detection of all activations for 4 as well as 7 runs from the simulated dataset. Since the time course of fASL data is highly redundant, the inclusion of the temporal information is very effective. This improvement in spatio-temporal denoising compared to spatial Gaussian denoising is illustrated in Figure 3 showing the acquired PWIs at 4 different time points for subject 4. Figure 4 shows the activation maps (FWE, p<0.05) of subject 4 and the corresponding fitted CBF response in the voxel with the highest t-value (blue arrow). Compared to standard spatial Gaussian denoising the ICTGV denoised time-series exhibits substantial improvement in noise-suppression. This results in statistically significant activations in the motor areas. Figure 5 shows exemplary the activation maps (FWE, p<0.05) for subject 1-3.Conclusion
The proposed method combines two spatio-temporal weights, allowing for stronger temporal regularization in areas with low dynamic change and weaker temporal regularization in areas with high dynamic change. The incorporation of temporal information in the denoising procedure improves the image quality and accomplished statistics for both, simulations and in-vivo data. This can be explained by exploiting the high temporal redundancy of fASL images directly in the denoising process. Group level analysis with more subjects will be part of future work.Acknowledgements
This work was funded by the Austrian Science Fund "SFB 3209-18".
NVIDIA Corporation Hardware grant support.
References
1. Detre JA, Leigh JS, Williams DS, et al. Perfusion imaging. Magn Reson Med. 1992;23(1):37-45
2. Williams DS, Detre JA, Leigh JS, et al. Magnetic resonance imaging of perfusion using spin inversion of arterial water. Proc Natl Acad Sci U S A. 1992;89(1):212-6.
3. Raoult H, Petr J, Bannier E, et al. Arterial spin labeling for motor activation mapping at 3T with a 32-channel coil: reproducibility and spatial accuracy in comparison with BOLD fMRI. Neuroimage. 2011;58(1):157-67.
4. Schloegl M, Holler M, Schwarzl A, et al. Infimal Convolution of Total Generalized Variation Functionals for dynamic MRI. Magn. Reson Med. 2017;78(1):142-155.
5. Luh WM, Wong EC, Bandettini PA, et al. QUIPSS II with thin-slice TI1 periodic saturation: a method for improving accuracy of quantitative perfusion imaging using pulsed arterial spin labeling. Magn Reson Med. 1999;41(6):1246-1254.
6. Bibic A, Knutsson L, Ståhlberg F, et al. Denoising of arterial spin labeling data: wavelet-domain filtering compared with Gaussian smoothing. MAGMA. 2010;23(3):125-137.
7. Hernandez-Garcia L, Jahanian H, Rowe DB. Quantitative analysis of arterial spin labeling FMRI data using a general linear model. Magn Reson Imaging. 2010;28(7):919-27.
8. Wang Z, Aguirre GK, Rao H, et al. Empirical optimization of ASL data analysis using an ASL data processing toolbox: ASLtbx. Magn. Reson. Imaging 2008;26:261–269.
9. Wang Z. Improving cerebral blood flow quantification for arterial spin labeled perfusion MRI by removing residual motion artifacts and global signal fluctuations. Magn. Reson. Imaging 2012;30:1409–1415.
10. Lu H, Donahue MJ and van Zijl PC. Detrimental effects of BOLD signal in arterial spin labeling fMRI at high field strength. Magn. Reson Med. 2006;56(3):546-52.
11. Chambolle A, Pock T. A First-Order Primal-Dual Algorithm for Convex Problems with Applications to Imaging. J. Math. Imaging Vis. 2010;40:120–145.
12. Buxton RB, Frank LR, Wong EC, et al. A general kinetic model for quantitative perfusion imaging with arterial spin labeling. Magn. Reson. Med. 1998;40(3):383–396.
Figures