2128
Abnormal growth trajectories of white matter in spontaneously hypertensive rats when compared to non-hypertensive controls: Implications for small vessel disease progression1Yale University, New Haven, CT, United States, 2University of Copenhagen, Copenhagen, Denmark, 3University of Rochester, Rochester, NY, United States
Synopsis
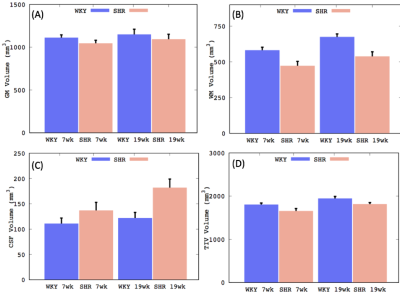
The spontaneously hypertensive rat (SHR) is a clinically relevant animal model in studying small vessel disease. Whole brain morphological differences between SHR compare to normotensive Wistar-Kyoto (WKY) rats were evaluated in parallel with development of chronic hypertension. Voxel-wise deformation based morphometry indicated progressive enlargement of the cerebral ventricles in SHR compare to WKY, and a fraction of the body and splenium of corpus callosum in SHRs were significantly smaller in the middle-aged rats but not in young-aged rats.
Introduction
Small vessel disease (SVD), or cerebral microvascular disease, in middle aged and elderly populations poses a significant risk for cognitive impairment, dementia, stroke and physical disabilities [1-2]. In SVD penetrating arterioles undergo progressive thickening of the wall, accompanied by inflammation, edema, glial scarring and demyelination. SVD is common in hypertensive patients, and animal models of chronic hypertension are essential to study to gain mechanistic insight into the pathophysiological progression of SVD. The spontaneously hypertensive rat (SHR) model, is a clinically relevant animal SVD model as it shares common pathological features with clinical cases of chronic hypertension including brain atrophy [3-4]. To our knowledge no systematic characterization of brain morphometry has been carried out in SHR rats in comparison to controls (WKY rats) in parallel with development of the fulminant hypertensive phenotype. Here we performed VFA-SPGR 3D imaging technique to characterize whole brain morphological differences between WKY and SHR in two age groups: 7 week old (mild hypertension) and 19 week old (chronic hypertension) using deformation based morphometry.Method
All imaging acquisitions were performed on a Bruker 9.4T/30 MRI instrument equipped with a volume transmit and surface receive coils interfaced with Paravision 5. WKY: (N=11) 7weeks (N=8) 19 weeks and SHR rats: (N=8) 7 weeks (N=9) 19-weeks; were anesthetized with Dexmedetomidine supplemented with isoflurane in an Air:O2 mixture and allowed to breathe spontaneously. A 3D VFA-SPGR technique was implemented using 3D spoiled gradient echo sequences (TR/TE/FA=15ms/4ms/2~30° 0.24x0.24x0.26mm) taken at multiple flip angles for B1+ correction as described previously [5]. 3D proton density weighted (PDW) images were calculated from the VFA-SPGR using a linear least square fit. Each PDW image was segmented into three tissue compartments (GM, WM, and CSF) using a custom made SHR-WKY population averaged tissue probability maps, created by manually editing the publicly available WKY template [6]. Segmented images were spatially normalized using the SPM12 DARTEL algorithm [7-8] and the Jacobian determinant was calculated in each voxel from the deformation field. Following 0.6mm spatial Gaussian smoothing, voxel-wise detection of morphological differences between the groups were detected using a t-test with the total intracranial volume (TIV) as a covariate.Results
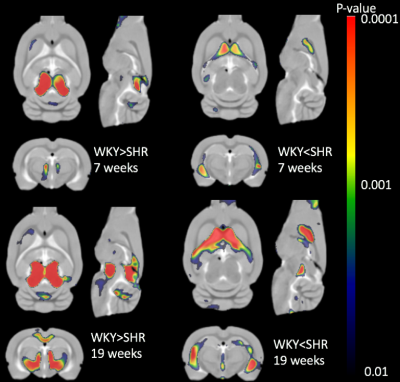
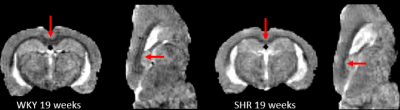
Our custom tissue probability maps yielded substantially more accurate segmentation results when compared to the WKY only template. Total tissue volumes, defined by the sum of the tissue probability maps, were calculated for grey matter (GM), white matter (WM) and CSF (Fig.1). Increasing GM, WM, and CSF volumes were documented in both strains as a consequence of brain growth (i.e. from 7-19 weeks) but tissue growth in SHRs was impaired when compared to WKYs. Spatially resolved volumetric differences analyzed by DBM yielded significantly (P<0.05) reduced volumes in the mid-brain in addition to enlarged CSF volumes in SHR compared to WKY in both age groups as shown in Fig. 2. Importantly, white matter, splenium and body of corpus callosum as shown in Fig. 3, were significantly smaller in SHR compared to WKY in the 19 week old group but not at 7 week old.Conclusion
In this study, PDW anatomical images were used to characterize morphological differences between SHR and WKY rats. Manual editing of the publicly available WKY tissue probability maps was necessary because a gross underestimation of CSF volume was observed in spite of deformable template to match the individual scans during the segmentation. Custom tissue probability maps were therefore created and both total tissue volumes and local volumetric differences were accurately evaluated. Our new observations emphasize the importance of considering TIV as a confound when analyzing volumetric differences and may help to explain abnormal CSF fluid flow in SHR rats [9-10]. The voxel-wise deformation field analyses highlighted progressive enlargement of the cerebral ventricles from 7-19 weeks. A fraction of the body and splenium of corpus callosum in SHRs were significantly smaller in the middle-aged rats, most likely a consequence of evolving hypertension interfering with myelination [11].Acknowledgements
NIH-R01AG048769
NIH-1R01NS100366
NIH-RF1AG053991
Leducq Foundation.
References
[1] Pantoni L. Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol 2010;9(7):689-701.
[2] Farrall AJ, Wardlaw JM. Blood-brain barrier: ageing and microvascular disease--systematic review and meta-analysis. Neurobiol Aging 2009;30(3):337-352.
[3] Ritter S, Dinh TT. Progressive postnatal dilation of brain ventricles in spontaneously hypertensive rats. Brain Res 1986;370(2):327-332.
[4] Nitkunan A, Lanfranconi S, Charlton RA, Barrick TR, Markus HS. Brain atrophy and cerebral small vessel disease: a prospective follow-up study. Stroke 2011;42(1):133-138.
[5] Lee H, Mortensen K, Sanggaard S, Koch P, Brunner H, Quistorff B, Nedergaard M, Benveniste H. Quantitative Gd-DOTA uptake from cerebrospinal fluid into rat brain using 3D VFA-SPGR at 9.4T. Magn Reson Med 2017.
[6] Valdes-Hernandez PA, Sumiyoshi A, Nonaka H, Haga R, Aubert-Vasquez E, Ogawa T, Iturria-Medina Y, Riera JJ, Kawashima R. An in vivo MRI Template Set for Morphometry, Tissue Segmentation, and fMRI Localization in Rats. Front Neuroinform 2011;5:26.
[7] Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage 2007;38(1):95-113.
[8] Ashburner J, Friston KJ. Unified segmentation. Neuroimage 2005;26(3):839-851.
[9] Kristian Nygaard Mortensen, Simon Sanggaard, Hedok Lee, Palle Koch, Maiken Nedergaard, Bjørn Quistorff, and Helene Benveniste. 3D DCE-MR imaging shows compromised brain waste transport in spontaneously hypertensive rats. Abstract: 4133 ISMRM Hawaii, USA, 2017
[10] Ringstad G, Vatnehol SAS, Eide PK. Glymphatic MRI in idiopathic normal pressure hydrocephalus. Brain 2017;140(10):2691-2705.
Figures