2127
Kinase-inactive Met mice show altered forebrain functional connectivity: A resting state functional MRI study1Diagnostic Radiology and Nuclear Medicine, University of Maryland School of Medicine, Baltimore, MD, United States, 2Anatomy and Neurobiology, University of Maryland School of Medicine, Baltimore, MD, United States
Synopsis
MET, the gene encoding tyrosine kinase receptor for hepatocyte growth factor, is a susceptibility gene for autism spectrum disorder (ASD). Genetically altered mice with a kinase-inactive Met offer a potential model for understanding neural circuit organization changes in autism. We employed resting-state functional MRI to a kinase-inactive Met mouse model to test our hypothesis that aberrant functioning of the somatosensory-thalamocortical system is at the core of the conspicuous somatosensory behavioral phenotypes observed in autism. Results showed impaired organization of large-scale network and increased somatosensory-thalamocortical connectivity with a sex dependent manner and differences between heterozygous and homozygous Met-Emx1 mice.
Purpose
MET, the gene encoding tyrosine kinase receptor for hepatocyte growth factor, is a susceptibility gene for autism spectrum disorder (ASD). Genetically altered mice with a kinase-inactive Met, targeted to the cerebral cortex and hippocampus, offer a potential model for understanding neural circuit organization changes in autism. We used this animal model to test the hypothesis that aberrant functioning of the somatosensory-thalamocortical system is at the core of the conspicuous somatosensory behavioral phenotypes observed in Met-Emx1 mice.Methods
Total of six groups (wild-type male and female, heterozygous Met-Emx1 male and female, homozygous Met-Met-Emx1 male and female) with 12 mice in each group were studied. All animal procedures conformed to the NIH guidelines and were approved by the University of Maryland, Baltimore, Institutional Animal Care and Use Committee.
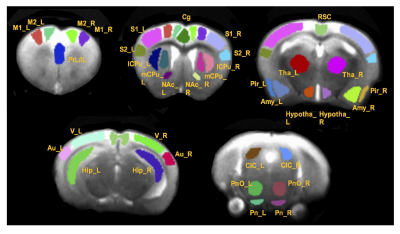
In vivo MRI experiments were performed on a Bruker BioSpec 7T MR scanner (Bruker BiospinMRI GmbH, Germany). Anesthesia was induced with isoflurane (~ 1 %) in oxygen enriched air. An MR compatible small-animal monitoring and gating system (SA Instruments, Inc., New York, USA) was used to monitor the animal respiration rate and body temperature. The animal body temperature was maintained at 36-37.5 °C using a warm water bath circulation. rsfMRI was acquired matching the anatomic images using a single shot echo planar imaging sequence (TR/TE = 1000/27.6 ms) with a 1.75 cm field of view (FOV) and an in-plane resolution of 273 μm2 using 18 slices at 1 mm thickness. Six hundred repetitions were taken twice, resulting in a scanning time of around 10 min for each dataset. All rsfMRI image preprocessing and processing were conducted using SPM12 (http://www.fil.ion.ucl.ac.uk/spm/) and AFNI (http://afni.nimh.nih.gov/afni). Thirty-seven regions of interest (ROIs) were manually defined based on a mouse brain atlas1 as shown in Fig. 1. Graph theory analysis was performed in MATLAB (Ver. R2014a, MathWorks, Inc.) based program graph-analysis toolbox (GAT) (https://www.nitrc.org/projects/gat/). Two-way ANOVA was performed on graph measures with genotype and sex as two factors. Measures that demonstrated a significant genotype x sex interaction were subjected to one-way ANOVA and genotype effect within males and females were assessed. Regionally-averaged-time course of somatosensory cortices (S1 and S2 combined) was extracted from each mouse and correlated with the time courses of other voxels in the whole brain to create a correlation map with somatosensory cortex as the seed ROI. Correlation maps were then converted to z-score connectivity maps with Fisher’s z transformation and were subjected to a one-way ANOVA (3dANOVA) analysis with males and females separated. AFNI function 3dFWHMx was used to determine the spatial smoothness of error variance which was used in 3dClustSim function to estimate the required minimum cluster size to maintain a 5% type 1 error rate.
Results
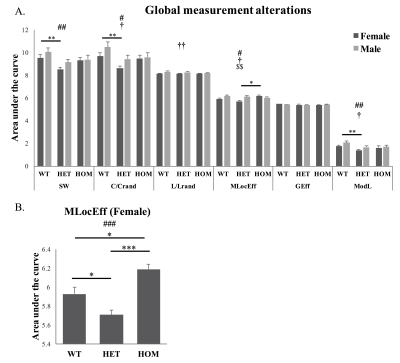
Network topology alteration
The areas under the curve of global network measures in a selected range of density were significantly different with respect to genotype (Fig 2A) in small-worldness (SW), normalized clustering coefficient (C/Crand) and modularity (ModL). The values were significantly reduced in heterozygous Met-Emx1 mice when compared to wild-type animals. Significant genotype x sex interaction was observed in local efficiency (MLocEff). A main effect of genotype was observed in female (Fig 2B). MLocEff was significantly increased in female homozygous Met-Emx1 mice and decreased in heterozygous Met-Emx1 mice compared to wild-type mice.
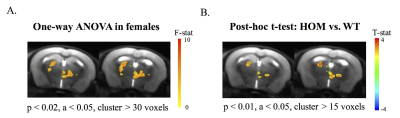
Alteration of somatosensory-cortex-thalamus functional connectivity
As revealed from functional connectivity map with somatosensory cortex as seed ROI, clusters in thalamus with significant main effects of genotype were only observed in females and was demonstrated in Fig 3A. In addition, between group comparison shown increased somatosensory-cortex-thalamus connectivity in female homozygous Met-Emx1 mice when compared with wild-type female mice (Fig 3B).
Discussion
The reduced network modularity, small-worldness and normalized clustering coefficient in the current study agree with previous studies involving young autism patients2, 3. The present results demonstrated that cortical and hippocampal disruptions of HGF–Met signaling during the brain maturational process may impair the maturation of large-scale network community. Hyperconnectivity has been observed in thalamocortical system of individuals with ASD4, 5. Enhanced somatosensory-cortex-thalamus connectivity in the present study provides evidence that the disruption of HGF–Met signaling in the neocortex during development can lead to functional deficit in the somatosensory inhibitory system and contribute to the autistic phenotype. Our findings also indicate that fully inactive of Met does not necessarily result in more alterations than single allele inactivation of Met. Also, the sex bias, which males are more prone to autism-associated impairments, may not hold true when focusing on a specific genetic basis.Acknowledgements
We are grateful to Dr. Rao Gullapalli for contributing to data interpretation. We thank Michelle Monroe and Shuxin Zhao for genotyping and breeding the mice. We also thank the Core for Translational Research in Imaging @ Maryland (C-TRIM) provided in vivo neuroimaging service for the study. This work was supported by the National Institutes of Health/ National Institutes of Neurological Disorders and Stroke (NS092216).References
- Paxinos G, Franklin K. The Mouse Brain in Stereotaxic Coordinates. 2012; 4th edn, Academic Press.
- Shi F, Wang L, Peng Z, et al. Altered modular organization of structural cortical networks in children with autism. PLoS One. 2004; 8, e63131, doi:10.1371/journal.pone.0063131.
- Barttfeld P, Amoruso L, Ais J, et al. Organization of brain networks governed by long-range connections index autistic traits in the general population. J Neurodev Disord. 2013; 5, 16, doi:10.1186/1866-1955-5-16.
- Nair, A. Carper RA, Abbott AE, et al. Regional specificity of aberrant thalamocortical connectivity in autism. Hum Brain Mapp 2015; 36: 4497-4511.
- Woodward, ND, Giraldo-Chica M, Rogers B, et al. Thalamocortical dysconnectivity in autism spectrum disorder: An analysis of the Autism Brain Imaging Data Exchange. Biol Psychiatry Cogn Neurosci Neuroimaging. 2017; 2: 76-84.
Figures