2124
Changes in corticospinal tract integrity in relation to recovery after cortical stroke as measured with DTI-based tractography in rat brain1Biomedical MR Imaging and Spectroscopy Group, Center for Image Sciences, University Medical Center Utrecht, Utrecht University, Utrecht, Netherlands, 2Neurological Disorders Research Centre, Qatar Biomedical Research Institute (QBRI), Hamad Bin Khalifa University, Doha, Qatar, 3Department of Chemistry and Earth Sciences, College of Arts and Sciences, Qatar University, Doha, Qatar
Synopsis
Preserved or restored integrity of the corticospinal tract (CST) is critical for motor recovery after stroke. However, data on spatiotemporal alterations in CST integrity after stroke are largely lacking. Here we implemented diffusion tensor-based tractography to identify the CST in rat brain, which we applied to measure microstructural changes along the CST following experimental stroke to the sensorimotor cortex. Number of tractography streamlines, fractional anisotropy (FA) and axial diffusivity (AD) were reduced 1 week post-stroke, and recovered to control levels after 28 weeks. This temporal pattern, reflective of white matter remodeling, coincided with loss and recovery of sensorimotor function.
Introduction
Recent studies in stroke patients have shown that preserved integrity of the corticospinal tract (CST) is critical for recovery of motor functions1. CST integrity may therefore provide an important predicting factor for patient-specific recovery potential, which can be assessed with diffusion tensor imaging (DTI)-based biomarkers2. However, (changes in) CST integrity may differ spatially and temporally after stroke to the brain’s motor network, which has hardly been investigated. This may be experimentally assessed in animal models of stroke that provide controllable and reproducible means to elucidate post-stroke recovery mechanisms. Therefore, the aims of the present study were to (1) implement a diffusion tensor-based tractography method to identify the CST in rat brain from in vivo diffusion-weighted images, and (2) to determine to what extent the CST is spatiotemporally affected in rats recovering from focal stroke to the sensorimotor cortex.Methods
Photothrombotic stroke was induced in the right sensorimotor cortex of adult male Sprague-Dawley rats3. Sensorimotor function was assessed with a forelimb-use asymmetry test4. Cross-sectional MRI (9.4T Varian MR system) was performed in control animals or after stroke at 1 week (n=5 control, n=7 stroke), 8 weeks (n=8 control, n=7 stroke) or 28 weeks (n=8 control, n=7 stroke). During MRI, rats were anesthetized and mechanically ventilated with 2.0% isoflurane in air/O2. Temperature was maintained at 37.0 ± 0.5 °C. After MRI, brains were harvested for immunohistochemistry and Fourier-transform infrared (FTIR) microspectroscopic imaging5. In vivo MRI consisted of anatomical imaging (BSSFP, 125 µm isotropic resolution) and diffusion-weighted (DW) imaging (4-shot 2D-EPI with diffusion-weighting with 2 b0 images and 30 directions with b = 1471 s/mm2, TR/TE=1700/34ms, 250x250x500µm3 resolution). Brain extraction from anatomical and DW images was performed with FSL’s Brain Extraction Tool (BET). DWI data were processed with FSL’s DTIFIT. After n3 inhomogeneity correction b0 images of each individual rat were registered to the b0 image of one reference rat (8 weeks, control) using the FSL Image Registration Tool (FLIRT). Conventional deterministic diffusion tensor (DT)-based whole-brain tractography was performed in MRtrix3 by generating 250.000 streamlines with a step size of 25 µm, fractional anisotropy (FA) threshold ≥ 0.3 and angle threshold of 30°. To select the left and right corticospinal tract (CST), first the bilateral internal capsule (IC) was manually outlined on a reference image. Inverse transformations were applied to register the bilateral IC back to the DWI data in individual animal space using FSL’s FLIRT. Second, two additional bilateral brain stem (approximately -7mm posterior from bregma) and optic fiber (approximately -1.5mm from bregma) regions were manually outlined for each individual animal, which served as “stop gate” and “not gate” respectively, whereas the IC served as “and gate” in the CST tract selection process. Along-tract analysis was performed to obtain the average axial diffusivity (AD), radial diffusivity (RD), mean diffusivity (MD) and FA along left and right CSTs.Results
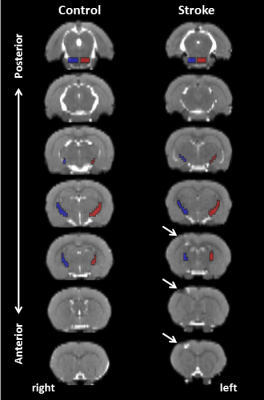
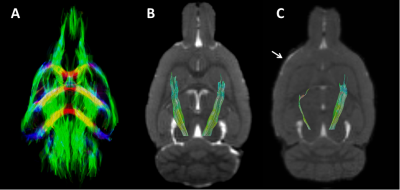
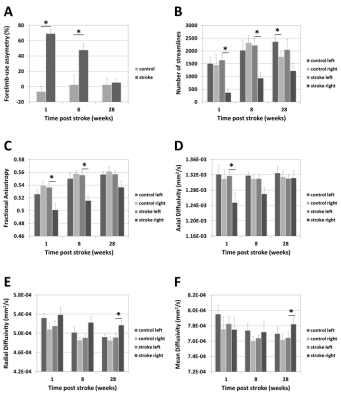
Photothrombotic stroke induced unilateral focal damage restricted to part of the sensorimotor cortex, characterized by signal abnormality on the anatomical images (Figure 1), without direct damage to the CST. The DT-based tract selection approach allowed specific selection of the bilateral CST in control and stroke rats (Figure 2). The extent and volume of tractography streamlines was clearly reduced in the ipsilesional CST after photothrombotic stroke (Figure 2C). Quantified DT-based tract parameters at different post-stroke time-points are shown in Figure 3. Sensorimotor function was significantly reduced at 1 week post-stroke, expressed by an increase in forelimb-use asymmetry due to increased use of the non-affected forepaw, which gradually recovered from 8 to 28 weeks after stroke (Figure 3A). This temporal pattern coincided with loss and recovery of the number of streamlines (Figure 3B), the FA (Figure 3C), and the AD (Figure 3D) along the ipsilesional CST. In contrast, ipsilesional RD (Figure 3E) and MD (Figure 3F) were significantly increased at 28 weeks.Discussion and conclusion
The diffusion tensor-based approach presented in this study successfully allowed specific selection of the bilateral corticospinal tracts (CSTs) in stroke-affected rat brain. Tract-specific analyses suggested microstructural changes in the ipsilesional CST remote from the cortical photothrombotic lesion territory, which may reflect widespread remodeling at the cellular level. The pattern of changes in diffusion scalars along the ipsilesional CST was similar to the pattern of alterations in sensorimotor function, suggesting that functional outcome after cortical stroke is closely associated with the microstructural status of the CST.Acknowledgements
This work was funded by NPRP grant no. NPRP-5-381-3-101
from the Qatar National Research Fund (a member of The Qatar
Foundation).
References
[1] Krakauer JW, Marshall RS, The Proportional Recovery Rule for Stroke Revisited. Ann Neurol. 2015 Dec;78(6):845-7.
[2] Lindenberg R, Renga V et al., Structural integrity of corticospinal motor fibers predicts motor impairment in chronic stroke. Neurology. 2010 Jan 26;74(4):280-7.
[3] Madinier A, Wieloch T et al., Impact of estrogen receptor beta activation on functional recovery after experimental stroke. Behav Brain Res. 2014 Mar 15;261:282-8
[4] Schallert T, Fleming SM et al., CNS plasticity and assessment of forelimb sensorimotor outcome in unilateral rat models of stroke, cortical ablation, parkinsonism and spinal cord injury. Neuropharmacology. 2000 Mar 3;39(5):777-87.
[5] Balbekova A, Lohninger H et al., Fourier Transform Infrared (FT-IR) and Laser Ablation Inductively Coupled Plasma-Mass Spectrometry (LA-ICP-MS) Imaging of Cerebral Ischemia: Combined Analysis of Rat Brain Thin Cuts Toward Improved Tissue Classification. Appl Spectrosc. 2017 Jan 1:3702817734618
Figures