2119
Protective effect of high creatine diet during chronic hepatic encephalopathy in young rats, an in vivo longitudinal 1H and 31P MRS study1Laboratory of Functional and Metabolic Imaging (LIFMET), Ecole Polytechnique Fédérale de Lausanne (EPFL), Lausanne, Switzerland, 2Neurometabolic Unit, Service of Clinical Chemistry, University Hospital of Lausanne, Lausanne, Switzerland, 3Swiss Center for Liver Disease in Children, University Hospitals Geneva, Geneva, Switzerland, 4Centre d’Imagerie Biomédicale (CIBM), Ecole Polytechnique Fédérale de Lausanne (EPFL), Lausanne, Switzerland
Synopsis
Chronic hepatic encephalopathy(CHE) is a serious neuropsychiatric disease with altered neurological status and changes in brain metabolites (among others, decrease in brain tCr). If CHE is acquired in childhood these conditions might perturb normal brain development. Our aim was to test whether oral Cr supplementation dampens the neurometabolic changes observed in CHE in a longitudinal model of chronic liver disease in young rats. Using in vivo longitudinal brain 1H and 31P-MRS, we showed rescued tCr levels, enhanced energy metabolism (restoration of ATP), improved antioxidant capacity (increased Asc), positive effect on phospholipid metabolism and smaller increase in Gln (marker of CHE).
Introduction
Chronic hepatic encephalopathy (CHE) is a serious neuropsychiatric disorder in adults and children and is due to chronic liver disease (CLD). CLD leads to increased toxins in the blood (e.g. ammonium, bilirubin), is followed by altered cognition, motor performance and changes in brain metabolites (increased Gln, decreased osmolytes, neurotransmitters, antioxidants and tCr)1–3 which are more severe if CHE is acquired during brain development. A decrease in tCr was also shown in developing rat brain-cells aggregates after ammonium exposure, possibly due to downregulation of Cr synthesis.4 It is known that decreased tCr can have a serious impact on the brain development in various diseases.5 Moreover, ammonium-induced impaired axonal growth was shown to be rescued by Cr treatment in organotypic 3D brain cell cultures.6 In addition, ammonium exposure induces expression of SLC6A8(Cr transporter) in astrocytes feet4, suggesting increased blood-brain barrier permeability to Cr (which is normally limited) in hyperammonemic conditions including CLD. Therefore we hypothesized that high Cr diet might be beneficial in CHE in young animals.
Our aim was to test whether oral Cr supplementation dampens the neurometabolic changes observed in CHE in a longitudinal model of CLD acquired in childhood.
Methods
Wistar male rats underwent bile duct ligation (BDL, model of CLD and CHE) or sham surgery at 21 days post-natal (p21) (equivalent to 1year old human in terms of brain development) and were divided into 4 groups: BDL(n=12), BDL+Cr(n=7), sham(n=9), sham+Cr(n=6). High Cr diet was given in a dose of 4g/kgrat/day.
1H-MRS, blood sampling and Open field test for motor activity were performed at week 6 and 8 after BDL. 31P-MRS was performed only at week 6 in fewer rats(3-4 rats per group).
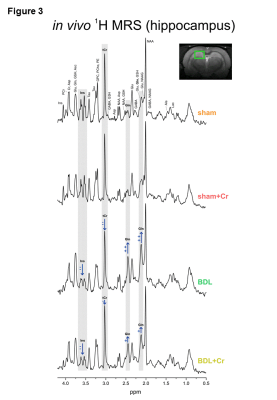
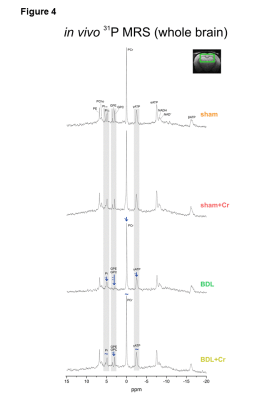
9.4T-system (Varian/Magnex Scientific) was used together with home built coils (1H-MRS: quadrature 1H-surface coil; 31P-MRS: quadrature 1H-loops with single 31P-loop) and FASTMAP7 for shimming. 1H-MRS spectra were acquired in hippocampus(2×2.8×2mm3) using SPECIAL8(TE/TR=2.8ms/4s,160avg.) and concentrations of metabolites were calculated by LCModel using water as reference. 31P-MRS spectra were acquired using a non-selective AHP pulse for excitation, localized by OVS(x,z) and 1D-ISIS(y) (TR=8s,384avg.), WALTZ-16 for NOE and 1H-decoupling in a VOI=5×9×9mm3. 31P-MR spectra were quantified by AMARES(jMRUI)9 and normalized for each rat using its PCr concentration from 1H-MRS in the same VOI.
Results and discussion
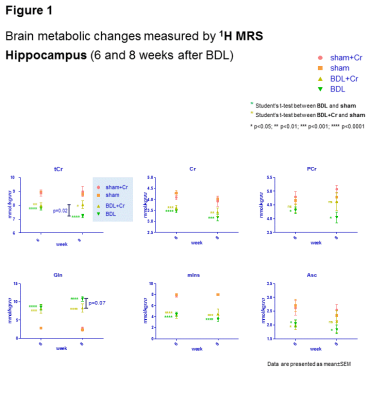
Eight weeks after BDL surgery, BDL pups with normal diet displayed increase in Gln (+368±29%,p<0.0001), known to increase in CHE due to ammonium detoxification. 1H-MRS also showed decrease in Ins (-57±4%,p<0.0001) as an osmotic compensation for Gln increase in astrocytes and decrease in other osmolytes as Tau (-17±2%,p<0.05), tCho (-60±4%,p<0.0001), decrease in neurotransmitters Glu (-18±4%,p<0.01) and GABA (-29±7%,p<0.05), in antioxidants Asc(-27±6%,p<0.05) and GSH(-38±6%,p=0.07), as well as in Cr(-20±4%,p<0.01) and PCr(-19±4%,p<0.01) resulting in -19±2%(p<0.0001) in tCr; all compared to shams at corresponding age (p-value represents Student’s t-test). As expected there was no difference in blood bilirubin, as marker of liver disease, between BDL and BDL+Cr. In addition, only a non-significant trend of improved motor activity was observed in BDL+Cr rats.
Some of the neurometabolic changes were improved by Cr diet in BDL+Cr: tCr (+11±4% higher in BDL+Cr than BDL rats,p<0.05), Gln (increased -24±14% less in BDL+Cr than BDL rats,p=0.07) and Asc (+15±13% higher in BDL+Cr than BDL rats,p=ns) resulting in no significant difference between BDL+Cr and shams for Asc(Fig1). There were no differences in the following metabolites between BDL and BDL+Cr: tCho, Tau, Glu and GABA.
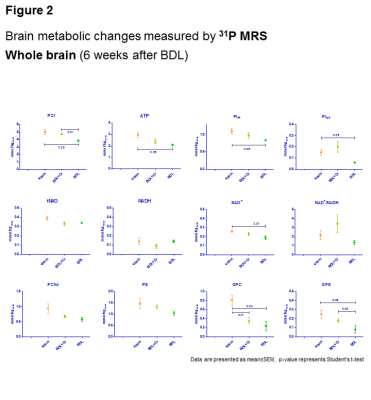
Oxidative stress is a known feature of CLD.10 No decrease in Asc in BDL+Cr compared to BDL rats might be due to decreased oxidative stress or increased brain capacity to regenerate its antioxidant sources. Improvement in brain Asc and not GSH in BDL+Cr is in agreement with previously proposed neuronal protection of Cr during high ammonium exposure observed in vitro6, as Asc is considered to be a neuronal antioxidant, while GSH is more specific of the glial compartment.11 Preliminary 31P-MRS data in BDL rats showed decrease in ATP(-29±3%,p=0.05), intracellular Pi(-24±2%,p=0.08), extracellular Pi(-59±4%,p=0.08) and NAD+(-24±7%,p=0.07) while in BDL+Cr rats there was no significant decrease of these metabolites (Fig2). Phospholipid metabolism was also perturbed in BDL rats with significant decrease of GPE(-68±16%,p=0.08) and GPC(-70±13%,p=0.04), consistent with tCho decrease observed by 1H-MRS. This was partially corrected in BDL+Cr with significant increase in GPE compared to BDL(Fig2).
Conclusions
High Cr diet in young cirrhotic rats partially rescued tCr levels in the brain and enhanced energy metabolism as showed by the restoration of ATP levels. This probably increased the brain capacity to regenerate oxidized Asc while it also restored GPC involved in phospholipid metabolism. The high Cr diet also contributed to lower BDL-induced Gln increase in CNS.Acknowledgements
Supported by CIBM of the UNIL, UNIGE, HUG, CHUV, EPFL, the Leenaards and Jeantet Foundations. EU: FP7-PEOPLE-2012-ITN project 316679 TRANSACT. The SNSF project no 310030_173222/1.References
1. Cudalbu C, Rackayova V, McLin VA, Braissant O. Brain Glutamine, Osmolytes and Edema in a Model of Chronic Hepatic Encephalopathy: in vivo and Londitudinal Measurements using 1H MRS, DTI and Immunofluorescence. In: The 16th ISHEN meeting, London. 2014.
2. Rackayova V, Braissant O, McLin V, Berset C, Lanz B, Cudalbu C. 1H and 31P magnetic resonance spectroscopy in a rat model of chronic hepatic encephalopathy: in vivo longitudinal measurements of brain energy metabolism. Metab Brain Dis [Internet]. 2016 Dec 9;31(6):1303–14. Available from: http://link.springer.com/10.1007/s11011-015-9715-8
3. Rackayová V, Braissant O, McLin VA, Cudalbu C. Chronic Hepatic Encephalopathy in the developing and adult rat brain: an in vivo non-invasive and longitudinal metabolic investigation using 1H MRS, DTI and immunohistochemistry. In: Proc Intl Soc Mag Reson Med 22. 2014. p. 808.
4. Braissant O, Cagnon L, Monnet-Tschudi F, Speer O, Wallimann T, Honegger P, et al. Ammonium alters creatine transport and synthesis in a 3D culture of developing brain cells, resulting in secondary cerebral creatine deficiency. Eur J Neurosci. 2008;27(August 2007):1673–85.
5. Rackayova V, Cudalbu C, Pouwels PJW, Braissant O. Creatine in the central nervous system: From magnetic resonance spectroscopy to creatine deficiencies. Anal Biochem [Internet]. 2017 Jul;529:144–57. Available from: http://linkinghub.elsevier.com/retrieve/pii/S000326971630389X
6. Braissant O, Henry H, Villard A-M, Zurich M-G, Loup M, Eilers B, et al. Ammonium-induced impairment of axonal growth is prevented through glial creatine. J Neurosci. 2002;22(22):9810–20.
7. Gruetter R, Tkác I. Field mapping without reference scan using asymmetric echo-planar techniques. Magn Reson Med [Internet]. 2000 Feb;43(2):319–23. Available from: http://www.ncbi.nlm.nih.gov/pubmed/10680699
8. Mlynárik V, Gambarota G, Frenkel H, Gruetter R. Localized short-echo-time proton MR spectroscopy with full signal-intensity acquisition. Magn Reson Med [Internet]. 2006 Nov [cited 2013 Nov 10];56(5):965–70. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16991116
9. (http://www.mrui.uab.es/mrui)
10. Bosoi CR, Rose CF. Oxidative stress: A systemic factor implicated in the pathogenesis of hepatic encephalopathy. Metab Brain Dis. 2013;28:175–8.
11. Rice ME. Ascorbate regulation and its neuroprotective role in the brain. Trends Neurosci. 2000;23(5):209–16.
Figures