2118
Chronic Oral Methylene Blue Treatment in a Rat Ischemic Stroke Model1Radiology and Preclinical Imaging Center, Stony Brook Medicine, Stony Brook, NY, United States, 2Loma Linda University, Loma Linda, CA, United States
Synopsis
Methylene blue (MB), an FDA-grandfathered drug, has been shown to reduce MRI-defined infarct volume in acute ischemic stroke. However, the efficacy of chronic MB treatment in stroke remains unknown. The goal of this study was to investigate the efficacy of chronic oral MB administration in ischemic stroke using MRI and behavioral tests. We found chronic MB treatment reduced MRI-defined total lesion volumes and improved functional behavioral outcomes, as well as reduced sub-acute hyperperfusion and white-matter damage. Our findings, for the first time, suggest that long-term MB oral administration is safe and has positive therapeutic effects in chronic stroke.
Introduction
Stroke is a leading cause of death and chronic disability worldwide (Benjamin et al., 2017). The available treatments for ischemic stroke remain very limited. There are currently no clinically approved neuroprotective drug for treatment of acute stroke. Methylene blue (MB), an FDA-grandfathered drug, has been used to treat methemoglobinemia and cyanide poisoning (Schirmer et al., 2011). We have previously demonstrated that acute treatment with a single-dose MB ameliorates behavioral deficits and reduces MRI-defined infarct volume in a transient (Shen et al., 2013) ischemic stroke models. Although these studies have shown that MB is neuroprotective for acute ischemic stroke, the efficacy of chronic MB treatment on stroke recovery in the chronic phase remains unknown. The goal of the current study is to test the long-term effects of chronic MB oral treatment on ischemic stroke in rats, using a randomized, double-blinded and vehicle-controlled design along with longitudinal MRI scan and behavioral tests.Methods
Transient (60-minutes) focal cerebral ischemia was induced by intraluminal filament middle cerebral artery occlusion (MCAO). In a randomized double-blinded, vehicle-controlled design, male Sprague-Dawley rats were randomly assigned to vehicle control or MB treatment groups after stroke. The gel food with food dye or MB solution (4mg/kg) was provided from 1 day to 21 days post stroke.
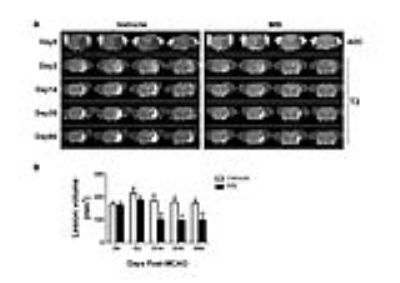
MRI was performed on a Bruker 7-T BioSpec Scanner. MRI (Diffusion-weighted images, CBF, T2-weighted images) was acquired at 30 mins after MCAO, and again on 2, 14, 35 and 60 days after MCAO. Sensorimotor function by the foot-fault test was evaluated on the same days preceding MRI scanning in the animals. ADC, CBF, T2, FA maps were generated to evaluate the stroke-induced brain damage and therapeutic effect of MB chronic treatment. Repeated-measures ANOVA and unpaired two-tailed t-tests were used to analyze the difference between vehicle- and MB-treated groups.
Results
The MRI-defined lesion volumes before treatment and day 2 after stroke were not significantly different between groups. By contrast, lesion volumes of the MB-treated group were significantly lower than the vehicle group at day 14 to 60. (100.5±12.5 vs. 184.0±26.3, P=0.022 on day 14; 96.6±5.8 vs. 177.3±22.1, P=0.016 on day 35; 97.7±14.7 vs. 172.4±17.2 P=0.049 on day 60; Two-way ANOVA, Fig.1). Also, compared to vehicle group, the foot faults in the MB-treated group were significantly lower on day 7 through day 60. CBF was significantly lower in MB-treated group on day 14, compared to the vehicle group. The corpus callosum (CC) volume was higher after MB-treatment compared to vehicle in the ipsilesional hemisphere (50.14±3.6 vs. 36.2±3.6, P=0.028, t-test) at day 60 after stroke.Discussion
The current studies extended previous acute MB treatment studies as followed: (1) Using daily oral chronic MB administration (day 1 to 21) and a double-blinded experimental design, we further demonstrated that the improvement in infarct volumes and functional recovery persisted at least up to 60 days, which was adequately covered the 30 days period, a Stroke Therapy Academic Industry Roundtable (STAIR) guideline suggested time windows for measuring the long-term therapeutic effect for stroke (Fisher et al., 2009). (2) Chronic low-dose MB reduced lesion volume starting from 14 days and persisted up to 60 days. The delayed neuroprotection effects of MB are consistent with the pattern of improvement on behavioral functions, which indicated the therapeutic effects of MB were delayed by this oral administration protocol. However, the mechanism of such delayed protective impact with chronic MB administration might be different from acute MB treatment. It is possible that acute MB treatment decreases infarct volume through reducing neural apoptosis and increasing autophagy (Jiang et al., 2015) by its unique antioxidant effect. Additional mechanisms (i.e., post-stroke neurogenesis) may be involved in chronic MB treatment. Recent evidence has shown that 5 days continuously intraperitoneal injection of 0.5mg/Kg MB increases cortical neurogenesis on 12 days after photothrombotic stroke (Ahmed et al., 2016). (3) We found that the extents of hyperperfusion in the initially defined core and mismatch tissue were reduced in the MB-treated group on day 2 and 14 after stroke. The possible mechanism for this is that MB may minimize the leakage of blood-brain-barrier (BBB) and dysfunction of vessels. Indeed, a study indicated that MB reduces BBB disruption in a pig cardiac arrest model (Miclescu et al., 2010).Conclusion
Long-term oral methylene blue treatment reduces brain lesion volume and white matter damage in the chronic phase of stroke, and improves behavioral function and regional CBF in transient focal ischemia in rats. These findings provided further supportive evidence that low-dose MB has positive therapeutic effects in the treatment of chronic stroke.Acknowledgements
This work was supported in part by NIH/NINDS (R01-NS45879).References
Ahmed, M.E., et al., 2016. Methylene Blue promotes cortical neurogenesis and ameliorates behavioral deficit after photothrombotic stroke in rats. Neuroscience. 336, 39-48. Benjamin, E.J., et al., 2017. Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association. Circulation. 135, e146-e603.
Fisher, M., et al., 2009. Update of the stroke therapy academic industry roundtable preclinical recommendations. Stroke. 40, 2244-50.
Jiang, Z., et al., 2015. The Effects of Methylene Blue on Autophagy and Apoptosis in MRI-Defined Normal Tissue, Ischemic Penumbra and Ischemic Core. PLoS One. 10, e0131929.
Miclescu, A., et al., 2010. Methylene blue protects the cortical blood-brain barrier against ischemia/reperfusion-induced disruptions. Crit Care Med. 38, 2199-206.
Schirmer, R.H., et al., 2011. "Lest we forget you--methylene blue...". Neurobiol Aging. 32, 2325 e7-16.
Shen, Q., et al., 2013. Neuroprotective efficacy of methylene blue in ischemic stroke: an MRI study. PLoS One. 8, e79833.
Figures