2115
Differential Effects of (+)MK801 and (-)MK801 on Brain Structure and Metabolism in Adolescence Rats As Revealed by VBM Analysis and In Vivo 1H-MRS1Wuhan National Laboratory for Optoelectronics, Huazhong University of Science and Technology, Wuhan, China, 2State Key Laboratory of Magnetic Resonance and Atomic and Molecular Physics, Wuhan Institute of Physics and Mathematics, Chinese Academy of Sciences, Wuhan, China
Synopsis
N-methyl-Daspartate receptor (NMDAR) antagonists, such as phencyclidine (PCP), ketamine and dizocilpine (MK801), have been widely used for inducing schizophrenia animal models. As a noncompetitive selective NMDAR antagonist, MK801 has two stereoisomers, (+)MK801 and (-)MK801, which have been found to induce different behavioral phenotypes and histological changes in animals. In this study, we compared differential effects of (+)MK801 and (-)MK801 on brain structure and metabolism in adolescence rats with MRI/in vivo 1H-MRS. The results showed that (+)MK801 induced more severe gray matter (GM) atrophy and more evident metabolic changes than (-)MK801, and the different effects were related to their potency at NMDA receptors.
Introduction
N-methyl-Daspartate receptor (NMDAR) hypofunction has been postulated to play a central role in the pathogenesis of schizophrenia1,2. Animal models of acute/chronic administration of NMDAR antagonist, such as phencyclidine (PCP), ketamine and dizocilpine (MK801), have been widely used for schizophrenia research3-5. MK801 has two stereoisomers, (+)MK801 and (-)MK801, with the latter being only one-seventh as potent as the former at the NMDA receptors6. It has been reported that (+)MK801 and (-)MK801, at the same dose, induced different behavioral phenotypes and histological changes7,8. In this study, we compared the MRI/in vivo 1H-MRS phenotypes in adolescent rats subjected to repeated treatments of (+)MK801 and (-)MK801, respectively.Materials and Methods
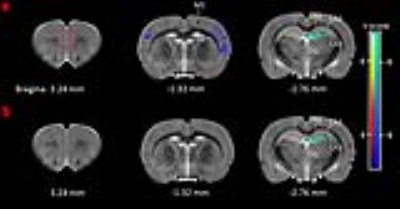
Adolescence male Sprague-Dawley (SD) rats received daily intraperitoneal injection of either (+)MK801 (n=10) or (-)MK801 (n=15) at a dose of 0.5 mg/kg body weight for 6 consecutive days, starting at postnatal day 30. The control animals of the same age were treated with the same amount of saline. MR experiments were performed 7 days after the last injection on a 7.0 T/20cm Bruker Biospec scanner with a volume coil for RF transmission and a quadrature surface coil for detection. The animals were anesthetized with 1.8-2.5% isoflurane. T2-weighted anatomical images were acquired with a RARE sequence, TR/ TEeff 2764/40 ms, RARE factor 4, matrix size 256×128, FOV 30×30 mm, slice thickness 0.8 mm, 20 contiguous coronal slices and 8 averages. VBM analysis was used to measure gray matter (GM) volume changes across the whole brain. Statistical significance level was set to p < 0.005, uncorrected, cluster size =50. In vivo 1H-spectra were acquired from bilateral medial prefrontal cortex (mPFC , 2.5 mm ×2.4 mm ×2 mm, red rectangle in Fig. 1) with a PRESS sequence, VAPOR water suppression, TR/TE 4000/15 ms, spectral bandwidth 4 kHz, 2048 data points and 512 averages. LCModel was used for quantification with the unsuppressed water signal as the internal reference. Only the results with CRLBs less than 35% were analyzed and reported. Independent sample Student’s t-test was used for statistical analysis. An FDR corrected p<0.05 was considered to be statistically significant.Results
VBM analysis (Figure 1) showed that (+)MK801 treatment induced widespread GM atrophy, involving mainly primary motor cortex (M1), primary somatosensory cortex (S1) and cornuammonis region 3 (CA3) of the hippocampus. In comparison, (-)MK801 treatment resulted in GM atrophy only in CA3. Figure 2 plots the changes in absolute concentration of myo-inositol (Ins), N-acetyl aspartate (NAA) and total creatine (tCr) in the mPFC. The (+)MK801 group showed significantly increased Ins and tCr concentrations over the control, while the (-)MK801 group had no statistically significant difference compared to the control group.Discussion
The results showed that, at the same dose, the (+)MK801 treatment resulted in more severe GM atrophy than the (-)MK801 treatment, probably attributable the pronounced neuronal degeneration (+)MK801 produced3,9. Interestingly, the (+)MK801-treated animals showed significantly increased Ins and tCr concentrations in the mPFC, even in the absence of regional GM atrophy. Similar effects, however, were not observed in the (-)MK801 group. The increased Ins and tCr concentrations in the mPFC of (+)MK801-treated animals could be a manifestation of gliosis in the region10,11.Conclusion
Repeated (+)MK801 treatment in adolescence rats induced more severe GM atrophy and more evident metabolic changes in the mPFC, relative to (-)MK801 treatment. It is concluded that the effects of MK801 on brain structure and metabolism are related to its potency at NMDA receptors.Acknowledgements
Supported by National Basic Research Program of China (2011CB707802), and Natural Science Foundation of China (21221064 and 81000598).References
1. Moghaddam B, Javitt D. From revolution to evolution: the glutamate hypothesis of schizophrenia and its implication for treatment. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 2012;37:4-15.
2. Kantrowitz JT, Javitt DC. N-methyl-d-aspartate (NMDA) receptor dysfunction or dysregulation: the final common pathway on the road to schizophrenia? Brain Res Bull 2010;83:108-21.
3. Lim AL, Taylor DA, Malone DT. Consequences of early life MK-801 administration: long-term behavioural effects and relevance to schizophrenia research. Behavioural brain research 2012;227:276-86.
4. Bubenikova-Valesova V, Horacek J, Vrajova M, Hoschl C. Models of schizophrenia in humans and animals based on inhibition of NMDA receptors. Neuroscience and biobehavioral reviews 2008;32:1014-23.
5. Mouri A, Noda Y, Enomoto T, Nabeshima T. Phencyclidine animal models of schizophrenia: approaches from abnormality of glutamatergic neurotransmission and neurodevelopment. Neurochemistry international 2007;51:173-84.
6. Wong E, Kemp J, Priestley T, Knight A, Woodruff G, Iversen L. The anticonvulsant MK-801 is a potent N-methyl-D-aspartate antagonist. Neurobiology 1986;83:7104-8.
7. Del Pozo E, Barrios M, Baeyens JM. The NMDA receptor antagonist dizocilpine (MK-801) stereoselectively inhibits morphine-induced place preference conditioning in mice. Psychopharmacology 1996;125:209-13.
8. Geter-Douglass B, Witkin JM. Dizocilpine-like discriminative stimulus effects of competitive NMDA receptor antagonists in mice. Psychopharmacology 1997;133:43-50.
9. Wu H, Wang X, Gao Y, Lin F, Song T, Zou Y, et al. NMDA receptor antagonism by repetitive MK801 administration induces schizophrenia-like structural changes in the rat brain as revealed by voxel-based morphometry and diffusion tensor imaging. Neuroscience 2016;322:221-33.
10. Hajszan T, Leranth C, Roth RH. Subchronic phencyclidine treatment decreases the number of dendritic spine synapses in the rat prefrontal cortex. Biological psychiatry 2006;60:639-44.
11. Catts VS, Wong J, Fillman SG, Fung SJ, Shannon Weickert C. Increased expression of astrocyte markers in schizophrenia: Association with neuroinflammation. The Australian and New Zealand journal of psychiatry 2014;48:722-34.
Figures