2112
Physical exercise enhances adult cortical plasticity in neonatal hypoxic ischemic injured rats: Evidence by BOLD-fMRI and LFP electrophysiological recording1Department of Health Sciences and Technology, SAIHST, Sungkyunkwan university, Seoul, Republic of Korea, 2Center for Neuroscience Imaging Research, Institute for Basic Science (IBS), Suwon, Republic of Korea, 3BnH Research co.,Ltd, Goyang, Republic of Korea, 4Department of Radiology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Republic of Korea, 5Center for Molecular and Cellular Imaging, Samsung Biomedical Research Institute, Seoul, Republic of Korea, 6Department of Global Biomedical Engineering, Sungkyunkwan University, Suwon, Republic of Korea, 7Department of Physiology, Yonsei University Colleage of Medicine, Seoul, Republic of Korea
Synopsis
The developing brain has a powerful
ability to modify its own structure and function for recovery from injury in
efforts to compensate for loss of function1,2. In critical period, developing
brain has maximal neuronal synaptic connections so it is most amenable to
changes in response to external stimulus such as physical exercise3.
However, after critical period, neuronal synaptic connections are reduced, and
maintained at the reduced state3. Here, we demonstrate enhanced
neuroplasticity with physical exercise performed beyond critical period for
rats that are injured during critical period. We obtained the BOLD-fMRI response
and the interneuron activity with LFP electrophysiological recording.
Purpose
The importance of early intervention, not after the critical period, has been a target issue for therapeutic management. Recently, in post-critical period, thalamo-cortical inputs are involved for plasticity4. In this study, we demonstrate enhanced neuroplasticity with physical exercise performed beyond critical period for rats that are injured during critical period. We employed HI injured rat brains (injury occurred at p7 age), and the physical exercise was performed during p28~p69 ages. We obtained the BOLD-fMRI activation map with forepaw stimulation and the interneuron activity using LFP electrophysiological recording at p42 & p 63 ages.Materials and Methods
Animal Preparations: A total of 24 Sprague-Dawley rats (postnatal day7, 16-20g) were randomly divided into four groups: HI injured, non-exercise (n=6); HI injured, exercise (n=6); Sham, non-exercise (n=6); Sham, exercise (n=6). All rats were subjected to right common carotid artery (CCA) occlusion and subsequent hypoxic exposure to 8% O2 for 150 min (36℃) for hypoxic ischemic injury (Rice-vannucci model). Sham operated rats were subjected to only incision into the skin on the right CCA, which was then closed immediately after.
Physical exercise and behavior test: Physical exercise was performed with a rotarod (Rotarod performance test) 5 days per week for 5 min per each session, which began at 4th week after the injury until 9th week. For behavior test, all subjects were on the accelerating rotarod and measured the time of falling down once a week.
BOLD-fMRI experiment: All BOLD-fMRI data were acquired at 6wks and 9wks after the injury using Bruker 7T MRI scanner (Bruker Biospoin Billerica, MA, USA) equipped with an array head coil for receiving and quadrature birdcage coil for transmitting. Electrical stimulation was applied to each forepaw for BOLD-fMRI at a frequency of 12 Hz (pulse width= 1.0 ms, current= 1.4 mA). We used single-shot gradient echo EPI sequence using the following acquisition parameters; TE= 60 ms, TR= 1000 ms, flip angle= 45°, number of average=1, field of view= 30 (readout) ×15 (phase encoding) mm2, matrix size= 64×32, in-plane resolution= 469×469 μm2, slice thickness= 1.5 mm, number of slices = 5 coronal slices, number of repetition= 80.
Data Analysis: All preprocessing was performed using the Analysis of Functional NeuroImages (AFNI) and FMRIB Software Library (FSL) packages including slice timing correction, motion correction, temporal normalization, linear registration, spatial smoothing and spatial normalization. And voxel-wise cross correlation analyze was conducted between with the BOLD temporal time series data and the electric stimulation paradigm.
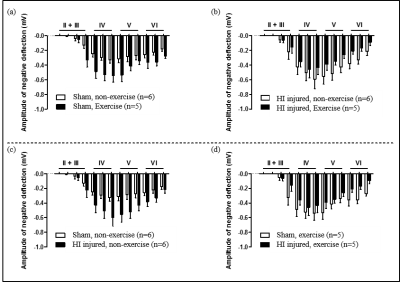
LFP electrophysiology recording: We obtained 100 pulses per layer and calculated the average for each layer. LFP was recorded at 9wks after the injury at 13 points: II + III: 50 µm / 200 µm / 350 µm / 500 µm, IV: 650 µm / 800 µm / 950 µm, V: 1100 µm / 1250 µm/1400 µm, VI: 1550 µm / 1700 µm / 1850 µm.
Results and Discussion
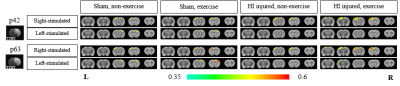
In our model, almost all one side of hemisphere including cortical and subcortical areas is subjected to severe neonatal HI brain damage producing a porencephalic cyst covering majority of the ipsilesional cortical brain structure (Fig. 1). In Figure 1, for both forepaw stimulations, BOLD-fMRI signal amplitudes were higher for exercise groups than non-exercise groups for HI injured rats: Increased BOLD signal activation was observed mostly in S1FL and M1 in the contralesional hemisphere for right forepaw stimulation (intact side) and mostly S1ULp and S1BF in the contralesional hemisphere with lower signal amplitude compared to the intact hemisphere for left forepaw stimulation (damaged side). Interesting to note, for sham operation groups of rats, exercise induced higher BOLD-fMRI signal activations. The LFP responses were well correlated with the BOLD-fMRI data, except for HI exercise groups, which may suggest a possibility of association of both inhibitory and excitatory interneurons for compensation of enhanced neuroplasticity (Fig. 2).Conclusion
We demonstrated the brain activation maps by BOLD-fMRI and LFP responses for the HI injured rat brains that were subjected to physical exercise after critical period. Enhanced neuroplasticity with physical exercise was clearly demonstrated on the intact side as well as the damaged side: Increased inter-hemispheric transfer and intra-hemispheric extension of activated areas were observed with physical exercise covering the widespread sensory-motor related areas in the contralesional hemisphere compared to the non- exercise groups.Acknowledgements
No acknowledgement found.References
1. Jung WB, et al. Neuroplasticity for spontaneous functional recovery after neonatal hypoxic ischemic brain injury in rats observed by functional MRI and Diffusion tensor imaging. Neuroimage. 2016 Feb 1; 126:140-50
2. Jhonston MV, et al. Plasticity in the developing brain: implications for rehabilitation. Dev Disabil Res Rev. 2009;15(2):94-101
3. Schinder, et al. The neurotrophin hypothesis for synaptic plasticity. Trends Neurosci, 2000. 23(12):p. 639-45
4. Chung, et al. Peripheral sensory deprivation restores critical-period-like plasticity to adult somatosensory thalamocortical inputs. Cell Reports 19, 2707-2717 June 27, 2017
Figures