2111
MRI detects neural protective effects of DAPT treatment at the subacute stage following cerebral ischemia1Radiology Deparment, Huashan Hospital of Fudan University, Shanghai, China, 2Huashan Hospital of Fudan University, Shanghai, China
Synopsis
Notch1 signaling plays time-dependent roles in the sequential process of neurogenesis after stroke. In this study, we aim to detect the appropriate therapeutic time frame of DAPT treatment based on the Notch1 signaling activation and NSCs responses after stroke. Combing the in vivo monitor of comprehensive microstructure changes with diffusion MRI and the in vitro analysis of neurogenesis and remyelination with immunohistology, we ultimately demonstrate the neurorestorative effects of DAPT treatment at the subacute stage after stroke. Our results suggested the appropriate therapeutic time window of inhibiting Notch1 signaling to maximally promote endogenous neurogenesis and axonal reorganization, which could enhance the efficacy of Notch-1 signaling related therapy and promote its application to clinical trials.
Introduction
Notch1 signaling, which is regarded as the potential therapeutic target for promoting functional neurological recovery after stroke1, plays time-dependent roles in the sequential process of neurogenesis2. The appropriate timely control of Notch1 signaling is thus required to augment neuronal progenitor pool and to promote neuronal differentiation to attain morphological and functional maturity in the adult brain3. In this study, we aim to detect the appropriate therapeutic time frame of DAPT treatment based on the Notch1 signaling activation and NSCs responses after stroke and measure the comprehensive microstructure changes with a set of MR parameters, combined with the post mortem histochemical analysis of neurogenesis and remyelination of corticospinal tract (CST), and ultimately demonstrate the neurorestorative effects of DAPT treatment at the subacute stage after stroke.Method
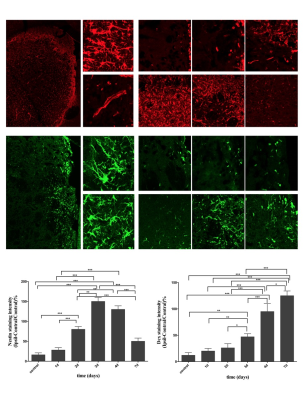
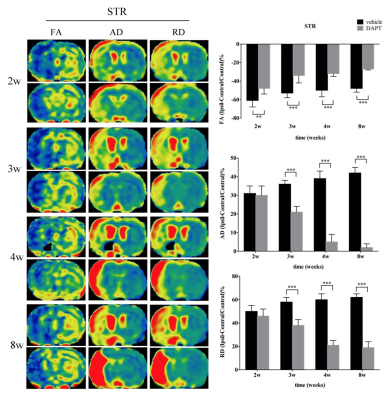
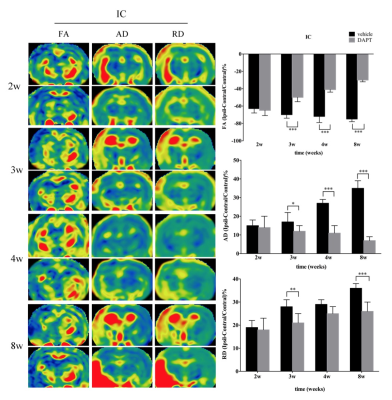
For quantitative evaluation of Notch1 signaling activation and neural proliferative response at the early stage of stroke, rats were subjected to middle cerebral artery occlusion (MCAO). Five rats were subsequently sacrificed before and 1 day, 2 days, 3 days, 4 days and 7 days after stroke, and subjected to immunohistochemical analysis of Nestin, Doublecortin (Dcx), Notch intracellular domain (NICD). To evaluate the proliferative response following treatment with the γ-secretase inhibitor at the subacute stage, rats were subjected to MCAO, treated by i.c.v administration (day 4) of DAPT or saline. Five rats of each subgroup were performed with magnetic resonance imaging (MRI) and subsequently decapitated for immunohistochemical analysis of Dcx, NeuN, MBP at 2 weeks, 3 weeks, 4 weeks and 8 weeks respectively.Results
In ischemic rats, NICD was detected in both the Nestin (+) and Dcx (+) cells in the ipsilateral STR. Both the number of NICD (+) cells and the intensity of Nestin reached the maximum at day3 (P < 0.05, vs. control, day1, day4, day7), while the intensity of Dcx was statistically elevated from day3 (P < 0.05, vs. control) following ischemia. Following DAPT treatment, compared with vehicle-treated rats, FA showed early and larger increase in STR and IC (both STR and IC, 2w-8w, P < 0.001). AD represented gradual decrease in STR (3w-8w, P < 0.001) and IC (3w, P < 0.05; 4w, 8w, P < 0.001). RD exhibited gradual decrease in STR (3w-8w, P < 0.001) and slower increase in IC (3w, P < 0.01; 8w, P < 0.001). Subsequent histology showed increased NeuN (+) cells in the ischemic boundary of STR (4w, P < 0.01; 8w, P < 0.001), as well as MBP (+) cells in both STR (4w, P < 0.01; 8w, P < 0.001) and IC (8w, P < 0.001).Discussion
In this study, firstly, we detected the location of NICD in Nestin-positive and Dcx-positive cells, indicating that Notch1 signaling is activated in this cell population and might regulate endogenous neurogenesis. Next, the temporal evolution of NICD indicating that Notch1 signaling was activated in the acute phase and then gradually declined in the subacute stage4. The temporal expression of Nestin showed the largest proliferative period of NSCs at 3 days, which is comparable to Notch1 signaling activation, and the significantly increase of Dcx from 3 days further indicated the potential transdifferentiation from NSCs to neuronal lineage. Adult neurogenesis following stroke is a sequential and multistep process4,5,6, in which, Notch1 signaling pathway mediates expansion of the neural progenitor pool and neuronal differentiation3. Based on these results, we blocked the Notch1 signaling with DAPT at day4 and monitored the morphological and functional changes of CST with diffusion MRI. The relative recovery of diffusion MR parameters in STR and IC mirrored the ameliorated integrity of axon bundles. The post-mortem histological examination confirmed the increased mature neurons in the ischemic boundary zone and the promoted remyelination across the CST.Conclusions
In this study, we provided the additional evidence that adult brain can use neuronal replacement from endogenous precursors to repair itself after stroke. Then with the non-invasive MR method, we detected the appropriate therapeutic time window of inhibiting Notch1 signaling to maximally promote endogenous neurogenesis and axonal reorganization. Our result could enhance the efficacy of Notch-1 signaling related therapy and promote its application to clinical trials.Acknowledgements
This study was supported by the grant of National Natural Science Foundation of China (No. 81371521) and Shanghai Natural Science Foundation of China (No. 09ZR1405100).References
- Wei Z, Chigurupati S, Arumugam TV, et al. Notch activation enhances the microglia-mediated inflammatory response associated with focal cerebral ischemia. Stroke 2011; 42(9):2589-94.
- Chambers CB, Peng Y, Nguyen H, et al. Spatiotemporal selectivity of response to Notch1 signals in mammalian forebrain precursors. Development 2001; 128(5):689-702.
- Wang L, Chopp M, Zhang RL, et al. The Notch pathway mediates expansion of a progenitor pool and neuronal differentiation in adult neural progenitor cells after stroke. Neuroscience 2009; 158(4):1356-63.
- Oya S, Yoshikawa G, Takai K, et al. Attenuation of Notch signaling promotes the differentiation of neural progenitors into neurons in the hippocampal CA1 region after ischemic injury. Neuroscience 2009; 158(2):683-92.
- Bonfanti L. PSA-NCAM in mammalian structural plasticity and neurogenesis. Prog Neurobiol 2006; 80(3):129-64.
- Christie KJ, Turnley AM. Regulation of endogenous neural stem/progenitor cells for neural repair-factors that promote neurogenesis and gliogenesis in the normal and damaged brain. Front Cell Neurosci 2012; 6:70.
Figures