2110
Vitamin B12 Deficiency Perturbed Energy Metabolism in Pre Frontol Cortex: 1H-[13C] NMR Study1NMR Microimaging and Spectroscopy, Centre for Cellular and Molecular Biology, Hyderabad, India, 2National Institute of Nutrition, Hyderabad, India
Synopsis
The consequences of severe deficiencies in micronutrients especially vitamin B12 on the developing brain during infancy and early post-natal period is not very clear. The current study aim to understand the effects of B12 deficiency on cognitive function using 1H-[13C]-NMR spectroscopy together with [1,6-13C2]glucose infusion in vitamin B12 deficient mice. Our findings indicate reduction in the metabolic activity of glutamatergic and GABAergic neurons in the prefrontal cortex of mice maintained with moderate and severe vitamin B12 defcient diet.
Introduction
Nutritional imbalance during early life acts as a physical stressor for the developing brain1. Vitamin B12 is one of the important micro-nutrients that is essential for brain development and function2. Deficiency in B12 has significant impacts on infant growth, cognition, social development, and depressive symptoms3. It has been shown that severe but not moderate vitamin B12 deficiency altered body composition, and induced adiposity in C57BL/6 mice4,5. However, impacts of vitamin B12 deficiency on brain brain energy metabolism is not understood. The objective of the current study is to evaluate the brain energy metabolism during moderate and severe vitamine B12 deficiency using C57BL6 mice.Methods and Methods
The animals protocol was approved by institutional animal ethics committee of National Institute of Nutrition, Hyderabad. Male C57BL mice (8 weeks) were randomly divided into three groups: Group A. AIN-76A control diet (n = 7, designated as Control); Group B. AIN-76A diet deficient in vitamin B12 with pectin as the source of fibre (n = 6, designated as Severe-B12R+); Group C. AIN-76A diet deficient in vitamin B12 with cellulose as the source of fibre (n = 6, designated as Moderate-B12R-). The mice also had ad libitum access to deionized water. After 4 months of feeding, the concentrations of plasma vitamin B12 was assessed, and the deficiency status was confirmed. The anxiety and depressive behaviors were assessed using Tail Suspension Test (TST), Forced Swim Test (FST), Novel Object Recognition Test (NORT) and Open Field Test (OFT). For metabolic analysis, prior-fasted mice were anesthetized using urethane (1.5 g/kg, i.p), and infused with [1,6-13C2]glucose for 10 minutes. Blood was collected from retro- orbital sinus for analyse plasma glucose concentration and 13C enrichment. The brain metabolism was arrested by using Focused Beam Microwave Irradiation (4.0 kW, 1.0 s)6. Brain were dissected and preserved in frozen condition for metabolic study. Metabolites were extracted from prefrontal cortex with slight modification of the previous protocol7. The concentration and 13C labelling of amino acids were measured in tissue extracts ex vivo in 1H-[13C]-NMR spectroscopy using Bruker 600MHz AVANCE III HD spectrometer8,9,10. One way Annova was performed to identify the statistical significance for the differences among different groups. All the results are presented as mean±standard deviation.Results and Discussion
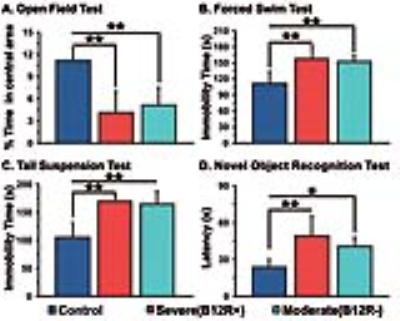
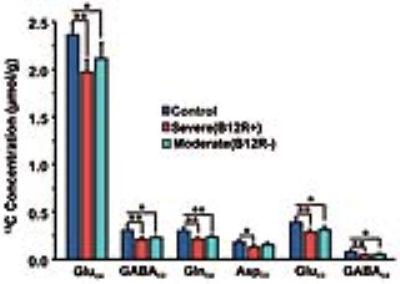
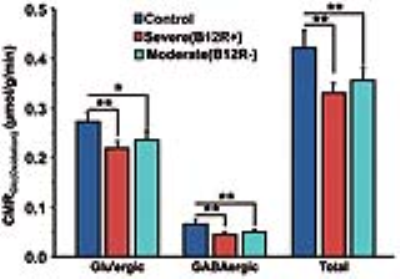
The deficiency in B12 leads to significant changes in the behavior of mice. There were significant increase in anxiety and depression like phenotype, and decrease in memory of mice having moderate (B12R-) and severe vitamin B12 deficiency (Figure 1). The level of glutamate was significantly reduced in both the groups (B12R- 14.1±0.2 µmol/g, n=6; B12R+ 14.1±0.5 µmol/g, n=6; Control 14.7±0.1 µmol/g, n=7, F[2,17], p=0.0059). There was no other difference in different metabolites among groups. The 13C labelling of GluC4, GABAC2, GlnC4 and GluC3 were decreased significantly in Severe (B12R+) and in Moderate (B12R-) deficient mice when compared with controls (Figure 2 & 3). This resulted in significant reduction in the metabolic activity of glutamatergic and GABAergic neurons in the prefrontal cortex of both deficient groups (Figure 4). These data together with established stoichiometric coupling between rates of neuronal glucose oxidation and neurotransmitter cycling suggests impaired neurotransmission in Vitamin B12 deficient mice.Conclusion
Although there are several literature are available regarding negative consequence of severe deficiency in micronutrients especially of vitamin B12 for the developing brain during infancy and early post-natal period, the mechanism for neuropsychiatric conditions like depression in adulthood is not very clear. The data from the current study provide the importance vitamin B12 for the normal brain development.Acknowledgements
This study was supported from funds from ICMR and CSIR-CCMB.References
1. Maniam J, Antoniadis C, Morris MJ. Early-Life Stress, HPA Axis Adaptation, and Mechanisms Contributing to Later Health Outcomes. Frontiers in endocrinology. 2014;5:73.
2. Stabler SP. Vitamins, homocysteine, and cognition. The American journal of clinical nutrition. 2003;78:359-60.
3. Maureen M. Black. Effects of vitamin B12 and folate deficiency on brain development in children. Food Nutr Bull. 2008 Jun; 29(2): S126–S131.
4. Ghosh S, Sinha JK, Kumar PU, Raghunath M. Severe but not moderate vitamin B12 deficiency impairs lipid profile, induces adiposity and leads to adverse gestational outcome in female C57BL/6 mice. Front Nutr; 3:1, 2016.
5. Ghosh S, Sinha JK, Muralikrishna B, Putcha UK, Raghunath M. Chronic transgenerational vitamin B12 deficiency of severe and moderate magnitudes modulates adiposity – probable underlying mechanisms. Biofactors; 43(3): 400-414, 2017.
6. Epstein et al (2013) Combinatorial assessments of brain tissue metabolomics and histopathology in rodent models of human immunodeficiency virus infection. J Neuroimmune Pharmacol 8:1224-1238.
7. Tiwari et al (2013) Glutamatergic and GABAergic TCA cycle and neurotransmitter cycling fluxes in different regions of mouse brain. J Cereb Blood Flow Metab 33:1523-1531.
8. de Graaf RA, Brown PB, Mason GF, et al. Detection of [1,6‐13C2]‐glucose metabolism in rat brain by in vivo 1H‐[13C]‐NMR spectroscopy. Magnetic resonance in medicine. 2003; 49(1):37-46.
9. Patel AB, Rothman DL, Cline GW and Behar KL. Glutamine is the major precursor for GABA synthesis in rat neocortex in vivo following acute GABA-transaminase inhibition. Brain research. 2001; 919(2): 207-220.
10. Fitzpatrick SM,
Hetherington HP, Behar KL and Shulman RG. The Flux from Glucose to Glutamate in
the Rat Brain in vivo as Determined by 1-Observed, 13C-Edited NMR
Spectroscopy. Journal of Cerebral Blood Flow & Metabolism.
1990; 10(2):170-179.
Figures