2109
Cerebral Reflections of Conditioned Pain Modulation in the Rat: An fMRI study1* Institute for Pharmacology and Toxicology, Friedrich-Alexander-University Erlangen-Nuremberg, Erlangen, Germany, 2Department of Radiology, University Hospital of the Friedrich-Alexander University Erlangen-Nuremberg, Erlangen, Germany
Synopsis
In this fMRI study we introduce an animal model to investigate the neural mechanisms of conditioned pain modulation (CPM) in rat brains. Here, a conditioning tonic cold (10°C water) stimulus at the right hindpaw was used to modulate nociceptive heat stimuli applied to the left hindpaw. Conditioned modulations in functional activation and related network connectivity could be observed in various brain structures involved in pain processing: brainstem and sensory input, lateral thalamus, sensorimotor cortex, frontal association cortex and limbic system. Additionally, over time decreasing resting state connectivity of brainstem and sensorimotor cortex due to cold water stimulation was found.
Introduction
Conditioned pain modulation (CPM) describes the phenomenon of a conditioning stimulus altering the pain perception of a noxious test stimulus1. Most CPM studies are psychophysically based human studies and do have clinical relevance in diagnostics and treatment2. However, the underlying neuronal mechanisms of CPM are not well understood. In animal models a comparable mechanism of endogenous pain inhibition was observed on the level of spinal neurons3. However, human EEG- and fMRI-studies demonstrated that CPM involves cortical brain areas4-6. Using fMRI we present an innovative animal model to investigate the involvement of specific brain areas of the central nervous system in CPM.Methods
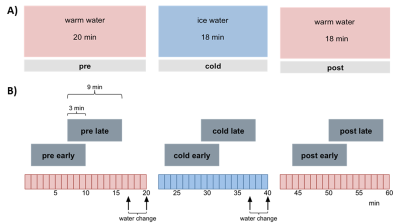
Experimental design: 9 male Wistar rats (anesthtetized with 1.2% isoflurane, body temperature 37°C) were scanned having their right hind paw placed in a custom-made cooling tube lined with a latex glove. Via barometric pressure warm or cold water was pumped into the tube. Each scan session consists of three continuous 20 min phases with alternating water temperatures: warm (30°C), cold (10°C), warm (30°C). During each phase six thermal stimuli were applied using a Peltier element at the left hind paw with alternating temperatures of innocuous 42 °C and noxious 52 °C. Each animal was measured twice: one first resting state scan without thermal stimulation and one second CPM scan with thermal stimulation (Fig. 1). A control group (n=9) was scanned identical to the experimental group described above but with three consecutive warm phases and no cold phase.
Image acquisition: MRI experiments were carried out using with a 4.7 T/40 cm horizontal bore actively shielded magnet BioSpec (BRUKER, Germany). Gradient system (200 mT/m) and whole-body birdcage resonator enabled homogenous excitation. An actively RF-decoupled 2x2 rat phased array head coil was used to acquire brain images. Resting state scans consisted of 1800 brain volumes acquired with a T2*-weighted single-shot gradient echo-based Echo Planar Imaging sequence (GE-EPI) covering 22 axial slices of the brain (TR= 2000 ms, TE=25.03 ms, in-plane resolution 391×391 μm, slice thickness 1 mm, matrix 64 ×64, FOV 25x25 mm, total time 60 minutes). At the end of each scan, 22 corresponding anatomical T2 reference images (RARE, slice thickness 1 mm, field of view 25×25 mm, matrix 256×256, TR = 3000 ms, TEef =11.7 ms, NEX=5) were acquired.
Data processing: Standard preprocessing was performed including interslice time, motion correction and spatial smoothing (for details see7). Stimulus driven fMRI data were temporally gaussian smoothed (FWHM 4s) and analyzed using GLM with 6 separate predictors, one for each stimulation temperature per session phase. The resulting statistical parametric maps (SPM) were FDR thresholded (q<0.05), and different groups of activated voxels were labeled as belonging to 181 pain related brain structures based on the rat atlas from Paxinos8. Subsequently, functional connectivity analysis was performed by cross correlating the residuals of the average time courses of the activated voxels per brain structure and predictor after global regression. Significant differences in connectivity strength for heat stimuli between session phases were calculated using paired t-test. Resting state fMRI data were subdivided longitudinal into two overlapping windows per phase (each 9 min with 3 min overlap, Fig. 1B). Each window per animal was low pass filtered <0.1 Hz and, after global regression, analyzed using a new approach combining classical seed correlation analysis and graph theory9,10.
Results and Discussion
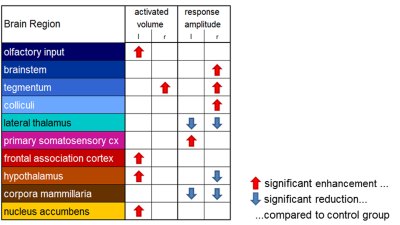
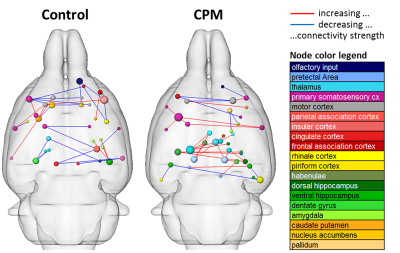
As expected, non-nociceptive heat stimulation (42°C) revealed no conclusive cold stimulation effects. However, the conditioning cold stimulus influences the neural processing of the nociceptive heat stimulus in various pain related brain structures (Figs. 2, 3). Contralateral to the heat stimulus enhanced response amplitudes in brainstem and tegmentum and reduced response amplitudes in hypothalamus were observed indicating modulation of the descending pathways in pain processing. Contralateral to the cold stimulus functional connectivity and response amplitudes of the sensory cortex were enhanced. The lateral thalamus, responsible for the sensory-discriminative dimension of pain, showed reduced response amplitudes but enhanced connectivity. Additionally, CPM modulations were observed in the frontal association cortex (the centre of the cognitive dimension of pain) and the limbic system (involved in emotional pain processing and learning).
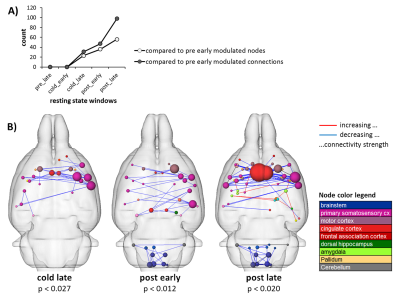
Resting state connectivity was modulated after the cold stimulus, with increasing extensiveness over time. Here, functional connectivity of the sensorimotor system, cingulum and brainstem were reduced whereas amygdala connections were enhanced (Fig. 4).
In conclusion, the introduced model is suitable to demonstrate the central effects of CPM in rats. The presented results contribute to the understanding of the underlying neural mechanisms of CPM.
Acknowledgements
This work was supported by BMBF Neurorad (02NUK034D)References
1. Yarnitsky D. Conditioned pain modulation (the diffuse noxious inhibitory control-like effect): its relevance for acute and chronic pain states. Curr Opin Anaesthesiol. 2010;23(5):611-5.
2. Nir RR, Yarnitsky D. Conditioned pain modulation. Curr Opin Support Palliat Care. 2015;9(2):131-7.
3. Le Bars D, Dickenson AH, Besson JM. Diffuse noxious inhibitory controls (DNIC). I. Effects on dorsal horn convergent neurones in the rat. Pain. 1979;6(3):283-304.
4. Moont R, Crispel Y, Lev R, Pud D, Yarnitsky D. Temporal changes in cortical activation during conditioned pain modulation (CPM), a LORETA study. Pain. 2011;152(7):1469-77.
5. Bogdanov VB, Vigano A, Noirhomme Q, Bogdanova OV, Guy N, Laureys S, et al. Cerebral responses and role of the prefrontal cortex in conditioned pain modulation: an fMRI study in healthy subjects. Behavioural brain research. 2015;281:187-98.
6. Youssef AM, Macefield VG, Henderson LA. Cortical influences on brainstem circuitry responsible for conditioned pain modulation in humans. Human brain mapping. 2016;37(7):2630-44.
7. Hess A, Axmann R, Rech J, Finzel S, Heindl C, Kreitz S, et al. Blockade of TNF-alpha rapidly inhibits pain responses in the central nervous system. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(9):3731-6.
8. Franklin KBJ, Paxinos G. The Mouse Brain in stereotaxic coordinates. Academic Press, New York, 3 Ed. 2008
9. Kreitz S, Heindl-Erdmann C, Axmann R, Zwerina J, Penninger J, Schett G, et al. Resting state networks in (transgenic) mice: differential effects of genetic background, sensory stimulation and pharmacological intervention. ISMRM 2011, Abstract No. 3685
10. Kreitz S, de Celis Alonso B, Uder M, Hess A. A new analysis of resting state connectivity and graph theory reveals distinctive short-term modulations due to whisker stimulation in rats. Neuroimage. Submitted.
Figures