2108
Understanding the Impact of Anesthetics on Neuronal and Astroglial Metabolic Activity using 1H-[13C]-NMR Spectroscopy1NMR Microimaging and Spectroscopy, Centre for Cellular and Molecular Biology, Hyderabad, India
Synopsis
Neurometabolic rate is coupled with neurotransmitter cycling, which is perturbed during various neurological disorders. Though, anesthetics are widely used in neurometabolic studies, their impacts on neural function is unclear. In the present study, effects of isoflurane and urethane on brain energy metabolism were investigated using 1H-[13C]-NMR spectroscopy in tissue extract during an infusion of [1,6-13C2]glucose or [2-13C]acetate. The reduction in neuronal metabolic activity under isoflurane was higher than urethane in the cerebral cortex and striatum. The data from the study indicate that impacts of anesthetics on neuronal function is more compared to astroglia suggesting that astroglial function is less affected with increased brain activity.
Introduction
Brain function is highly dependent on energy metabolism whose loss herald’s cognitive impairments, particularly in the aging and neurodegenerative diseases1. Anesthetics are routinely used in study of brain energy metabolism in different neurological conditions in rodents2. However, the impacts of anesthetics on neurometabolites homeostasis and neural metabolic activity is not well understood. Isoflurane and urethane are the commonly used anesthetics in animal studies. Isoflurane, a short acting inhalation anesthetic, is believed to decrease the gamma-aminobutyric acid type A receptors (GABAAR) activity3. On the other hand, urethane (ethyl carbamate) is long lasting anesthetic and believe to alter both excitatory and inhibitory signaling along with the ion channel activity4. The quantitative impacts of these anesthetics on neuronal and astroglial metabolic function is not clear in vivo. In the current study, we have evaluated the effects of isoflurane and urethane on the metabolic activity of neurons and astroglia in mice brain using 1H-[13C]-NMR spectroscopy together with an infusion of 13C labeled glucose and acetate.Materials and Methods
All animal experiments were performed under approved protocols by the Institutional Animal Ethics Committee. Three months old male C57BL6J mice were divided into following groups: Group A. Awake (n=6), Group B. Isoflurane (1.2-1.5% mixed in air), Group C. Urethane (1.5 g/kg, intraperitoneal)5. Animal were fasted for 4 hours prior to study. Mice were maintained under particular anesthetic (isoflurane or urethane) for 45 min followed by an infusion of either [1,6-13C2]glucose6 for 10 min or [2-13C]acetate7 for 15 min via tail vein to investigate neuronal or astroglial metabolic activity. Blood was collected from the retro orbital sinus for collection of plasma, and mice were euthanized using Focused Beam Microwave Irradiation at 4.9 kW for 0.85 sec8. The cerebral cortex and striatum were dissected, and metabolites were extracted9. The total concentration and percent 13C enrichment of metabolites were measured in tissue extracts using 1H-[13C]-NMR spectroscopy10. The cerebral metabolic rates associated with glutamatergic neurons, GABAergic neurons and astroglia were calculated from the 13C labeling of brain amino acids11. The statistical significance of difference between groups were determined by Student's t test.Results and Discussion
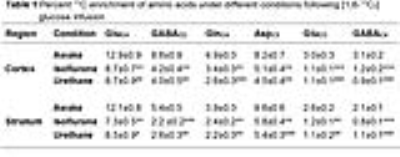
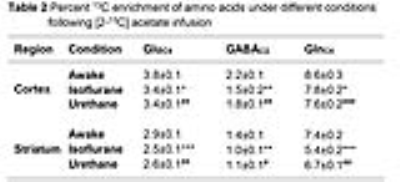
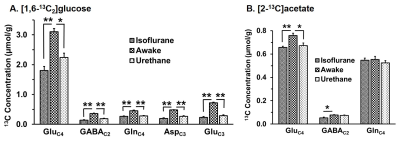
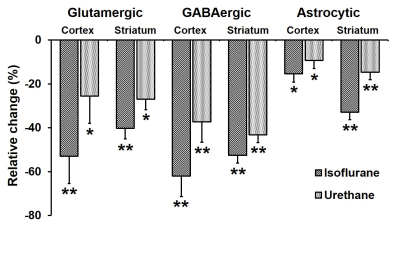
The levels of alanine, succinate and lactate were decreased in the cerebral cortex and striatum under urethane when compared with awake. In contrast level of alanine was increased significantly (p<0.05) while succinate and aspartate were decreased under isoflurane. The percent 13C labeling of metabolites from glucose and acetate was reduced significantly (p<0.05) in the cerebral cortex and striatum regions under both anesthetics (Table 1 and 2), which led to decrease 13C concentration of amino acids from [1,6-13C2]glucose and [2-13C]acetate (Figure 2A & 2B) in the cerebral cortex. A similar results were observed in the striatum. The cerebral metabolic rates of glucose oxidation by glutamatergic (Awake 0.41±0.05, n=6; Isoflurane 0.19±0.03, n=6, -53%; Urethane 0.23±0.11 µmol/g/min, n=6, -25%) and GABAergic neurons (Awake 0.11±0.01, n=6; Isoflurane 0.04±0.01, n=6, -62%; Urethane 0.05±0.02 µmol/g/min, n=6, -37%) were reduced significantly (p<0.01) in the cerebral cortex of mice anesthetized with isoflurane as well as urethane when compared with awake. A similar reduction in the metabolic rates were observed in the striatum. Moreover, the rates of acetate oxidation were decreased significantly (p<0.01) in the cerebral cortex (Awake 0.14±0.01,n=6; Isoflurane 0.12±0.01, n=6, -15%; Urethane 0.13±0.01 µmol/g/min, n=6, -9%) and striatum (Awake 0.11±0.01, n=6; Isoflurane 0.07±0.007, n=6, -32%; Urethane 0.09±0.005, n=6, -14%) in anesthetized mice (Figure 3). The reduction in neuronal metabolic activity under isoflurane was higher than urethane in the cerebral cortex and striatum. In contrast, astroglial activity was more perturbed in striatum. In general, the perturbation in neuronal metabolic activity is more than astroglia (Figure 3). These data indicate that impacts of anesthetics on neuronal function is more compared to astroglia suggesting that astroglial function is less affected with increased brain activity.Acknowledgements
Authors would like to thank Mr. Shoumik Roy, Mr. Sampath Kumar TK and Mr. Hari Prasanth for their help in conducting animal studies. The study was supported by funding from CSIR-CCMB.References
1. de Graaf et al (2004) Regional glucose metabolism and glutamatergic neurotransmission in rat brain in vivo. Proc Natl Acad Sci Usa 101:12700-12705.
2. Tiwari et al (2013) Glutamatergic and GABAergic TCA cycle and neurotransmitter cycling fluxes in different regions of mouse brain. J Cereb Blood Flow Metab 33:1523-1531.
3. Jiang Li et al (2012) Differential Effect of Isoflurane, Medetomidine, and Urethane on BOLD Responses to Acute Levo-Tetrahydropalmatine in the Rat. Magnreson Med. 68: 552-559.
4. Hara Koji et al (2002) The Anesthetic Mechanism of Urethane: The Effects on Neurotransmitter-Gated Ion Channels. Anesth Analg 94:313-318.
5. Bagga et al (2013) In Vivo NMR Studies of Regional Cerebral Energetics in MPTP Model of Parkinson`s Disease: Recovery of Cerebral Metabolism with Acute Levodopa Treatment. J. Neurochemistry 127:365-377.
6. Fitzpatrick et al (1990) The Flux from Glucose to Glutamate in the Rat Brain In Vivo as Determined by IH-Observed, 13e-Edited NMR Spectroscopy. J Cereb Blood Fow Metab 10:170-179.
7. Patel et al (2010) Evaluation of cerebral acetate transport and metabolic rates in the rat brain in vivo using 1H-[13C]-NMR.J Cereb Blood Fow Metab 30, 1200–1213.
8. Epstein et al (2013) Combinatorial assessments of brain tissue metabolomics and histopathology in rodent models of human immunodeficiency virus infection. J Neuroimmune Pharmacol 8:1224-1238.
9. Patel et al (2001) Glutamine is the major precursor for GABA synthesis in rat neocortex in vivo following acute GABA-transaminase inhibition. Brain Res. 919:207-220.
10. de Graaf et al (2003) Detection of [1,6-13C2]-Glucose Metabolism in Rat Brain by In Vivo 1H-[13C]-NMR Spectroscopy. Magn. Res. Med. 49:37-46.
11. Veeraiah et al (2014) Dysfunctional Glutamatergic and GABAergic Activities in Prefrontal Cortex of Mice in Social Defeat Model of Depression. Bio. Psychiatry 76:231-238.
Figures