Yukiko Masaki1, Yuto Kashiwagi1, Takemi Rokugawa1, and Kohji Abe1
1SHIONOGI & CO., LTD., Osaka, Japan
Synopsis
Pharmacological MRI allows the visualization of brain
pharmacological effects of drugs using fMRI. In order to clarify the
relationship between fMRI signal and receptor occupancy or behavioral response,
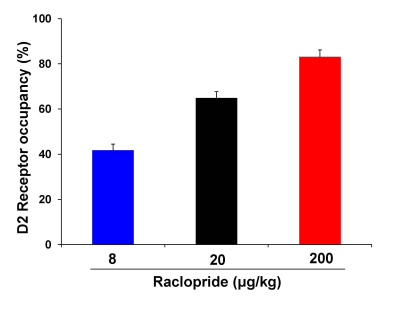
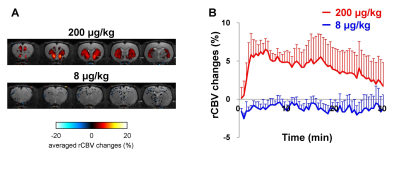
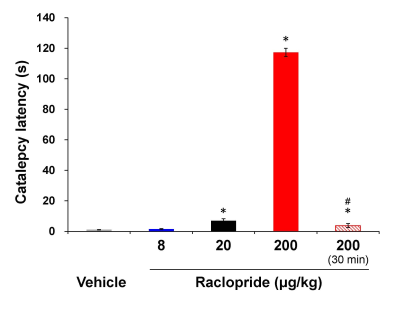
we performed [11C]-raclopride PET, fMRI and the behavioral
assessment with raclopride, dopamine D2 receptor antagonist. The positive fMRI
response and cataleptic behavior were observed at the dose of raclopride
showing 83% of D2 receptor occupancy, but not at the dose of raclopride showing
42% of D2 receptor occupancy. These results suggest that fMRI and behavioral
response induced by raclopride will be needed the high D2 receptor occupancy.
Acknowledgements
No acknowledgement found.References
1. Jonckers E, Shah D, Hamaide J, et al. The
power of using functional fMRI on small rodents to study brain pharmacology and
disease. Front. Pharmacol., 2015 Oct 21;6:231
2. Mandeville JB, Marota JJ, Kosofsky BE,
et al. Dynamic functional imaging of relative cerebral blood volume during rat
forepaw stimulation. Magn Reson Med. 1998 Apr;39(4):615-24
3. Sotoyama H, Zheng Y, Iwakura Y, et al. Pallidal
hyperdopaminergic innervation underlying D2 receptor-dependent behavioral
deficits in the schizophrenia animal model established by EGF. PLoS One.
2011;6(10):e25831