2104
Comparison of intravenous and intraperitoneal routes of Omniscan administration with respect to its retention in the rat brainSerguei Liachenko1, Natalya Sadovova1, Sherry Ferguson1, Joseph Hanig2, Merle G Paule1, Olayinka Dina3, Anthony Fotenos3, Adebayo Laniyonu3, and Ira Krefting3
1Neurotoxicology, NCTR / FDA, Jefferson, AR, United States, 2OTR, CDER / FDA, White Oak, MD, United States, 3DMIP, CDER / FDA, White Oak, MD, United States
Synopsis
Preclinical investigation into the brain retention of gadolinium contrast agents after repeated dosing requires extensive animal handling, particularly for intravenous injections. To decrease the potential stress caused by surgical implantation of intravenous catheters and constant maintenance of those catheters for repeated dosing, we proposed to administer Omniscan for such studies via the intraperitoneal route to laboratory rodents. After 20 dosed over 5 weeks, Omniscan retention was similar in both routes of administration.
Introduction
To date, only relatively short duration studies of gadolinium-based contrast agents (GBCA) administration have been investigated in laboratory rodents (e.g., 20 doses over 5 weeks) 1. However, it is important to study the effects of more prolonged exposures on GBCA retention and its effects on brain function and the potential neurotoxicity. Such studies would require protracted maintenance of surgically implanted intravenous (IV) catheters to effect repeated IV delivery of GBCAs with increasing likelihood of lost catheter patency, leading to lost data and the need for more animals. The intraperitoneal (IP) route of administration is much more easily accomplished and poses much less stress to the animals. While rapid GBCA delivery (via IV dosing as opposed to IP) is essential for dynamic studies, it may not be as critical for studying the long-term accumulation of GBCAs in the brain after multiple injections. Here, we compare both IV and IP administration of Omniscan for 5 weeks (20 doses total) on retention in the rat brain as well as on behavior and neurohistopathology.Methods
The animal use protocol was approved in advance by the NCTR IACUC. Male Sprague-Dawley rats (N = 18, 66 ± 1 days old, 325 ± 21 g) were used in this study. Twelve rats were surgically implanted at the vendor site (Charles River, Inc.) with femoral vein catheters with externalized injection ports (PinPort, Instech Laboratories, Inc). Animals received IV Omniscan (N = 6, 0.62 mmol/kg), IP Omniscan (N = 6, 0.62 mmol/kg), or IV saline injections (control, N = 6, 1.24 ml/kg) over 5 weeks (4 injections/week, 20 total). Brains were imaged before the start of treatment and weekly thereafter (6 scans/subject). MRI was performed using a 7 tesla Bruker Biospec AV III equipped with 12 cm ID gradient insert (440 mT/m) and 38 mm litz-cage quadrature RF coil (Doty Scientific, Inc). A quantitative T2 mapping spin echo sequence was used, as described in 2: TR = 6000 ms, TE = 15 ms, ETL = 12, MTX = 192 × 192, FOV = 3.84 × 3.84 cm, 28 slices, 1 mm slice thickness, acquisition time ~20 min. T2 maps were calculated off-line as described in 2. Before each MRI scan (including baseline), open field locomotor activity was assessed. At the end of the treatment, rats were perfused trans-cardially with 4% paraformaldehyde as described in 3 for follow-up histopathological evaluation. The regions of interest (ROIs), which delineated deep cerebellar nuclei (DCN) 4, were drawn manually on T2 maps. Statistical analysis was performed using repeated measures ANOVA.Results
Figure 1 shows an example of manual ROI positioning (white) on skull-stripped T2 maps. There were significant effects of Omniscan on T2 (F = 14.205, P = 0.002) and time (F = 3.597, P = 0.006) and the interaction between these factors was also significant (F = 3.392, P = 0.008). However, there was no significant effect of Omniscan administration route (F = 0.386, P = 0.548) on its retention in DCN. Figure 2 shows the dynamics of averaged T2 changes in DCN between animals after both IP and IV routes of Omniscan and saline administration. These data suggest that for long-term studies of GBCA retention in the rat brain IP injections can successfully replace IV and thus improve the efficiency and animal well-being in such experiments. Omniscan treatment did not cause significant changes in open field locomotor activity or any detectable histological brain abnormalities.Conclusion
Repeated dosing with Omniscan leads to its identical retention in the rat brain independently of the route of administration (IV or IP). Therefore, the use of the IP route for long-term GBCA experiments seems justified.Acknowledgements
This work was supported by the National Center for Toxicological Research (NCTR) and the Center for Drug Evaluation and Research (CDER), US FDA (protocol number E07625).References
- Olchowy, C., et al., The presence of the gadolinium-based contrast agent depositions in the brain and symptoms of gadolinium neurotoxicity - A systematic review. PLoS One, 2017. 12(2): p. e0171704.
- Liachenko, S. and J. Ramu, Quantification and reproducibility assessment of the regional brain T2 relaxation in naive rats at 7T. J Magn Reson Imaging, 2017. 45(3): p. 700-709.
- Hanig, J., et al., The use of MRI to assist the section selections for classical pathology assessment of neurotoxicity. Regul Toxicol Pharmacol, 2014. 70(3): p. 641-7.
- Robert, P., et al., T1-Weighted Hypersignal in the Deep Cerebellar Nuclei After Repeated Administrations of Gadolinium-Based Contrast Agents in Healthy Rats: Difference Between Linear and Macrocyclic Agents. Invest Radiol, 2015. 50(8): p. 473-80.
Figures
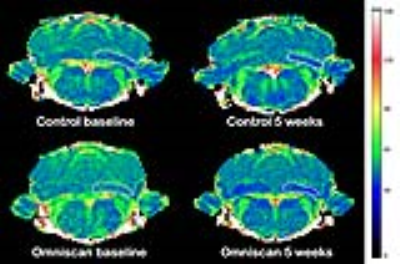
Figure 1. Representative T2 maps the same rats
before (left) and after the end of dosing (right). Manual ROIs (white) were drawn
on DCN on both sides of the brain (only one side is shown for clarity).
Figure 2. Dynamics
of T2 changes in the DCN of the rats treated with Omniscan IV
(green) and IP (red) and Saline IV (black). * - statistically
significant difference between treated and control groups (Bonferroni post-hoc
tests in RM ANOVA).