2100
MR neuroimaging and proton spectroscopy in Wolfram syndrome1Department of Biomedical and NeuroMotor Sciences, University of Bologna, Bologna, Italy, 2Functional MR Unit, Policlinico S.Orsola - Malpighi, Bologna, Italy, 3IRCCS Institute of Neurological Sciences of Bologna, Bologna, Italy
Synopsis
We characterized neurodegeneration in Wolfram syndrome by combining MR neuroimaging and proton MRS, and evaluated pathological accumulation of brain lactate as a. mitochondrial oxidative impairment marker. Cerebellar white matter loss was widespread, while grey matter loss was stronger within sensorimotor and cognitive cerebellar lobules. Infratentorial neurodegeneration was confirmed by biochemical signs of neuro-axonal degeneration in cerebellum and pons. The lack of abnormal ventricle lactate suggests an absence of dysfunction of mitochondrial metabolism. These morphological, microstructural and biochemical alterations were in line with neuropathological findings of loss of myelinated axons in the visual system, smaller brainstem and cerebellar white matter loss.
Introduction
Wolfram syndrome (WS) is a rare (1 in ~770000)1 progressive autosomal recessive genetic disease, characterized by early childhood onset of the combination of insulin dependent diabetes mellitus, optic nerve atrophy, diabetes insipidus and deafness. Neurologic abnormalities (cerebellar ataxia, brainstem dysfunction and epilepsy) are also present from the early stages1. The neurodegeneration pattern of this disorder has been characterised by few in vivo neuroimaging studies: decrease grey matter (GM) volume and white matter (WM) microstructural alterations were found mainly in the brainstem, cerebellum and optic radiations1,2. Changes in mitochondrial dynamics were observed in relation to a deficiency of WFS1 (protein Wolfram syndrome 1) and mitochondrial morphological and biochemical changes were detected at muscle biopsy3,4,5. The aim of this study was to further characterize Wolfram syndrome neurodegeneration, by combining MR neuroimaging and proton MRS, and evaluate pathological brain accumulation of lactate as a potential mitochondrial oxidative impairment marker.Methods
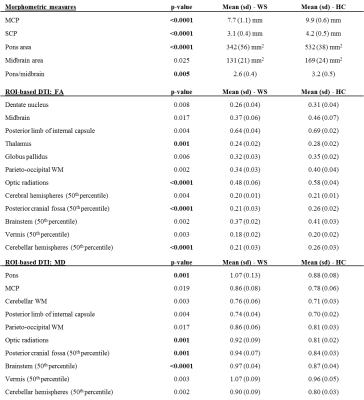
Ten patients with confirmed WFS1 gene mutations, who underwent brain MR in their diagnostic workup, were enrolled. Acquisitions were performed with a 1.5T GE scanner, equipped with a head coil. The standardized MR protocol included volumetric T1-w images (TR/TE/TI=12.5/5.1/600ms, 1mm3 isotropic), diffusion-weighted MRI (TR/TE=10.000/87.5ms, 25-directions, b-value=900 mm2s−1, voxel=1.25x1.25x4mm) and single voxel 1H-MR spectroscopy (PRESS) with localizations in the lateral ventricles (TR/TE=1500/288ms, volume=3.7-8.2ml, NEX=384), left cerebellar hemisphere (TR/TE=4000/35ms, volume=6ml, NEX=64), left parieto-occipital white matter (TR/TE=4000/35ms, volume=8ml, NEX=64) and pons (TR/TE=1500/40ms, volume=1.2-1.5ml, NEX=512)6,7. Not all the patients completed the whole protocol; groups of sex- and age-matched healthy controls were included (Tab.1). Voxel-based morphometry (VBM) analyses were performed on T1-w images using SPM 12.0. Whole-brain VBM was supplemented by software optimized for evaluating infratentorial structures (Spatially Unbiased Infratentorial Toolbox, SUIT8). Morphometric measurements were performed following previously reported methods9,10. Sagittal middle cerebellar peduncle (MCP) diameter, coronal superior cerebellar peduncle (SCP) diameter, pons and midbrain areas, MCP/SCP ratio, pons/midbrain ratio and MR Parkinsonism Index were evaluated. Diffusion-weighted data underwent standard pre-processing and tensor fitting. Voxelwise analysis of tensor parameters was performed using TBSS (Tract-Based Spatial Statistics). Mean Diffusivity (MD) and Fractional Anisotropy (FA) values were also evaluated within manually defined ROIs (listed in Tab.2). To globally evaluate brain DTI parameters, we created MD and FA histograms of cerebral and cerebellar hemispheres, brainstem, vermis and posterior fossa1. Spectra were analysed with LCModel 6.3, and relative metabolites concentrations were evaluated. Group comparisons were performed with univariate analyses (SPSS®); voxelwise comparisons were non-parametric (permutation method). Statistical significance was set to p<0.05 (corrected for multiple comparisons).Results
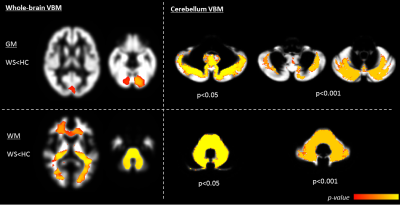
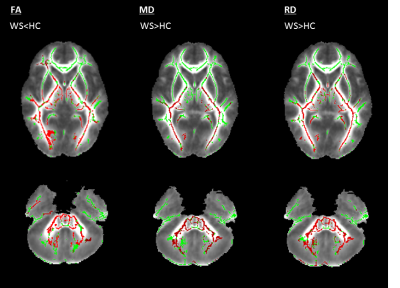
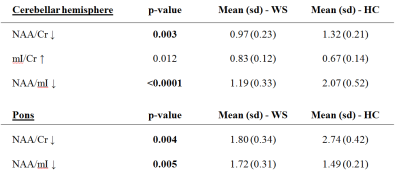
Whole-brain VBM showed GM loss in WS in the occipital pole and cerebellum (Fig.1) and WM loss in the corpus callosum, anterior corona radiate, optic radiations, cerebellum and brainstem (Fig.1). Cerebellum-specific VBM showed that WM loss was widespread while GM loss affected the whole cerebellum but with a stronger effect within left lobule VIII, vermis IX, bilateral crus I, II, V, VI. TBSS highlighted lower FA (and higher MD) for patients mainly within optic and thalamic radiations, corpus callosum and cerebellar tracts. These effects were mainly driven by a higher radial diffusivity, while axial diffusivity was unaltered (Fig.2). ROI-based DTI showed altered FA and MD mainly within the posterior fossa, basal ganglia and optic radiations (Tab.2). As for morphometric measures, patients had smaller MCP and SCP diameters, pons and midbrain areas, and a lower pons to midbrain area ratio (Tab.2). Ventricular lactate was absent in 7/10 patients, while traces were present in 3/10 patients. WS patients had lower cerebellar NAA/Cr and NAA/mI concentrations, and higher mI/Cr. Lower NAA/Cr and NAA/mI concentrations were also found in the pons, while no differences were found in the parieto-occipital white matter (Tab.3).Discussion and conclusions
Whole-brain VBM, TBSS and ROI-based DTI results are in line with previous literature1,2. Considering that all previous advanced neuroimaging studies in Wolfram syndrome report data on about 20 patients in total, this confirmation is significant. The most innovative aspects of this study were the fine-grained characterization of cerebellar neurodegeneration using both biochemical and topographic profiles. In particular, cerebellar VBM demonstrated that WM loss was widespread with analogous extent, while GM loss was stronger within cerebellar lobules involved in sensorimotor and cognitive/behavioural functions12,13. This might be of particular interest taking into account the psychiatric manifestations of Wolfram syndrome described in combination with neurological deficits14. Infratentorial neurodegeneration was also confirmed by biochemical signs of neuro-axonal degeneration in cerebellum and pons. The lack of abnormal ventricular lactate excludes the hypothesis of dysfunction in mitochondrial metabolism. These morphological, microstructural and biochemical alterations of the cerebellum are in line with the neuropathological investigation of two WS patients15,16.Acknowledgements
No acknowledgement found.References
1. Hershey T, Lugar HM, Shimony JS et al. Early brain vulnerability in Wolfram syndrome. PLoS One 2012;7(7):e40604.
2. Lugar HM, Koller JM, Rutlin J et al. Neuroimaging evidence of deficient axon myelination in Wolfram syndrome. Sci Rep. 2016 Feb 18;6:21167.
3. Bu X, Rotter JI. Wolfram syndrome: a mitochondrial-mediated disorder? Lancet. 1993 Sep 4;342(8871):598-600.
4. Bundey S, Poulton K, Whitwell H et al. Mitochondrial abnormalities in the DIDMOAD syndrome. J Inherit Metab Dis. 1992;15(3):315-9.
5. Cagalinec M, Liiv M, Hodurova Z et al. Role of Mitochondrial Dynamics in Neuronal Development: Mechanism for Wolfram Syndrome. PLoS Biol. 2016 Jul 19;14(7):e1002511.
6. Gramegna LL, Tonon C, Manners DN et al. Combined Cerebellar Proton MR Spectroscopy and DWI Study of Patients with Friedreich's Ataxia. Cerebellum. 2017 Feb;16(1):82-88.
7. Zanigni S, Testa C, Calandra-Buonaura G et al. The contribution of cerebellar proton magnetic resonance spectroscopy in the differential diagnosis among parkinsonian syndromes. Parkinsonism Relat Disord. 2015 Aug;21(8):929-37.
8. Diedrichsen J. A spatially unbiased atlas template of the human cerebellum. Neuroimage 2006 Oct 15;33(1):127-38.
9. Oba H, Yagishita A, Terada H et al. New and reliable MRI diagnosis for progressive supranuclear palsy. Neurology 2005 Jun 28;64(12):2050-5.
10. Quattrone A, Nicoletti G, Messina D et al. MR imaging index for differentiation of progressive supranuclear palsy from Parkinson disease and the Parkinson variant of multiple system atrophy. Radiology 2008 Jan;246(1):214-21.
11. Rizzo G, Tonon C, Valentino ML et al. Brain diffusion-weighted imaging in Friedreich's ataxia. Mov Disord. 2011 Mar;26(4):705-12.
12. Stoodley CJ, Schmahmann JD. Evidence for topographic organization in the cerebellum of motor control versus cognitive and affective processing. Cortex 2010 Jul-Aug;46(7):831-844.
13. O'Reilly JX, Beckmann CF, Tomassini V et al. Distinct and overlapping functional zones in the cerebellum defined by resting state functional connectivity. Cereb Cortex 2010 Apr;20(4):953-965.
14. Owen MJ. Psychiatric disorders in Wolfram syndrome heterozygotes. Mol Psychiatry. 1998 Jan;3(1):12-3. PubMed PMID: 9491807.
15. Hilson JB, Merchant SN, Adams JC et al. Wolfram syndrome: a clinicopathologic correlation. Acta Neuropathologica 2009;118, 415–428.
16. Shannon P, Becker L, Deck J. Evidence of widespread axonal pathology in Wolfram syndrome. Acta Neuropathol. 1999 Sep;98(3):304-8.
Figures