2092
Concept of Gadolinium-Ferritin Interactions as Explanation of Signal Intensity Changes in Deep Brain Nuclei after Application of Gadolinium-Based Contrast AgentsJosef Vymazal1, Jitka Neburkova2, Martin Dracinsky2, Mohan Pingle3, Petr Cigler4, and Aaron Michael Rulseh1
1Dept. of Radiology, Na Homolce Hospital, Prague, Czech Republic, 2Insittute of Organic Chemistry and Biochemistry, Prague, Czech Republic, 3Dept. of Radiology, Na Homolce Hospital, Praha, Czech Republic, 4Petr Cigler, Insittute of Organic Chemistry and Biochemistry, Prague, Czech Republic
Synopsis
Interaction between gadolinium-based contrast agents and metalloprotein ferritin may explain observed signal intensity changes in vivo due to T1 (and T2) shortening in the globus pallidus and dentate nucleus.
INTRODUCTION
Increased signal intensity on unenhanced T1-weighted MRI in subjects with a history of gadolinium-based contrast agent (GBCA) application was first reported in 2013, primarily in the globus pallidus and dentate nucleus, regions known to accumulate iron in the metalloprotein ferritin. We hypothesized that gadolionium-ferritin interactions may be responsible and aimed to determine whether gadolinium-ferritin complexes form under physiological conditions upon interaction of ferritin with various contrast agents in vitro, to measure their relaxation times, and to measure T1 signal intensity ratios and T2 relaxation times in the globus pallidus patients with numerous GBCA applications.MATERIALS AND METHODS
Five GBCA were analyzed: two linear agents (gadodiamide [Omniscan], gadobenate dimeglumine [Multihance]) and three macrocyclic agents (gadobutrol [Gadovist], gadoterate meglumine [Dotarem], gadoteridol [ProHance]). In addition, a non-clinical Gd-EDTA chelate was used. Gadolinium concentration was measured. A group of 14 subjects who underwent 7–50 (mean 26.9) GBCA applications were scanned with a routine protocol. T1 signal intensity ratios (region/CSF) and T2 relaxation times (dual-echo fast-spin-echo sequence) in the globus pallidus were evaluated.RESULTS
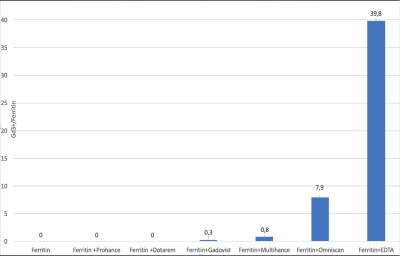
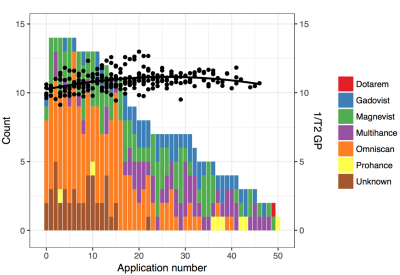
A differing number of gadolinium atoms were bound to each ferritin molecule depending on the GBCA used (Figure 1). Gadoterate meglumine (macrocyclic) 0 ions bound per ferritin molecule, gadoteridol (macrocyclic) 0 ions, gadobutrol (macrocylic) 0.3 gadolinium ions, gadobenate dimeglumine (linear) 0.8 ions, gadodiamide (linear) 7.9 ions, and the (non-clinical) Gd-EDTA chelate 39 gadolinium ions. T1 and T2 times of these complexes were shortened according to the number of gadolinium atoms bound. In vivo, a non-linear dependence of the signal ratio (T1) and 1/T2 on number of gadolinium-based contrast agent applications was observed (Figure 2).DISCUSSION
Our work establishes the formation of gadolinium-ferritin complexes upon interaction of GBCA with ferritin under physiological conditions in vitro and emphasizes the importance of chelate stability. Our data shows the presence of both T1- and T2-shortening in gadolinium-ferritin complexes in vitro, as well as in the globus pallidus in vivo. Our data suggest that signal intensity changes in the globus pallidus are of non-linear character.Acknowledgements
No acknowledgement found.References
1. T. Kanda, K. Ishii, H. Kawaguchi, K. Kitajima, and D. Takenaka, “High Signal Intensity in the Dentate Nucleus and Globus Pallidus on Unenhanced T1-weighted MR Images: Relationship with Increasing Cumulative Dose of a Gadolinium-based Contrast Material,” Radiology, vol. 270, no. 3, pp. 834–841, Dec. 2013.
2. R. J. McDonald et al., “Intracranial Gadolinium Deposition after Contrast-enhanced MR Imaging,” Radiology, vol. 275, no. 3, pp. 772–782, Mar. 2015.