2074
Nutritional intervention for developmental brain damage: neuroprotection with Lactoferrin following intrauterine growth restriction.1Service développement et croissance, Université de Genève, Geneva, Switzerland, 2Laboratoire d'imagerie fonctionnelle et métabolique, Ecole polytechnique fédérale de Lausanne, Lausanne, Switzerland, 3Institut translationnel d'imagerie moléculaire, Université de Genève, Geneva, Switzerland
Synopsis
Lactoferrin (Lf) is an iron-binding glycoprotein secreted in milk known as antioxidant, antimicrobial and anti-inflammatory. Infants exposed to adverse prenatal conditions of intrauterine growth restriction (IUGR), are at high risk for neurological morbidities. The aim of this work was to assess neuroprotective effect of Lf on brain microstructure by using diffusion imaging and NODDI model at 9.4T in a model of 50% gestational caloric restriction. Diffusion MRI derived parameters changes following IUGR were partially restored in the Lf supplemented group, providing evidence of a neuroprotective effect.
Introduction
Infants exposed to adverse prenatal conditions of intrauterine growth restriction (IUGR), are at high risk for neurological morbidities such as cerebral palsy, mental retardation, a wide spectrum of learning disabilities and developmental behavioral and neuropsychiatric disorders later in life [1]. Experimental studies of IUGR in the rat, induced by protein or caloric restriction, have shown extensive effects on brain development including white and grey matter structural changes [1]. Lactoferrin (Lf) is an iron-binding glycoprotein secreted in milk known as antioxidant, antimicrobial and anti-inflammatory [2]. In a previous work, we showed neuroprotective effects on the injured developing brains following hypoxia-ischemia [2] or inflammation [3] as well as on a model of IUGR induced by dexamethasone [4]. The aim of this work was to assess neuroprotective effect of Lf on brain microstructure by using diffusion tensor imaging (DTI) and neurite orientation density and dispersion imaging (NODDI) at 9.4T in a model of 50% gestational caloric restriction.Methods
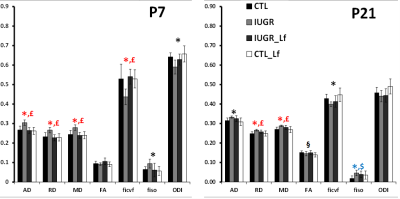
A model of 50% gestational caloric restriction (CR) was used. Four groups were designed and pregnant rats had either ad libitum access to food (control group, CTL, n=6 at P7 and n=7 at P21) or 50% of the controls’ intake (restricted group, IUGR, n=6 at P7 and n=4 at P21). Treated dams were supplemented with bovine Lf for controls (control-Lf group, CT_Lf, n=6 at P7 and n=7 at P21) and IUGR, (IUGR_Lf group, n=6 at P7 and n=4 at P21). The both diets (Lf and control diets) were isocaloric. MR experiments were performed on an actively-shielded 9.4T/31cm magnet (Agilent) equipped with 12-cm gradient coils (400mT/m, 120μs) with a 2.5 mm diameter birdcage coil. A multi-b-value shell protocol was acquired using a spin-echo sequence with the following parameters: FOV(mm2)/matrix size/number of slices/thickness (mm) = 20×20/96×64/12/0.6 at P7 and 27×27/128×64/14/0.6 at P21, axial slices, 3 averages with TE/TR = 45/2000 ms. A total of 96 DWI were acquired, 15 of them as b0 reference images. The remaining 81 were separated in 3 shells with the following distribution (# of directions/b-value in s/mm2): 21/1750, 30/3400 and 30/5100. All 81 directions were non-collinear and were uniformly distributed in each shell. Diffusivities (Mean, MD; Axial, AD and Radial, RD) and fractional anisotropy (FA) were derived from the tensor by using DTI-TK. Acquired data were fitted using the NODDI toolbox [5] leading to intra-neurite volume fraction (ficvf), cerebrospinal volume fraction (fiso) and orientation dispersion index (ODI). Three different brain regions were analyzed: cortex (Cx), external capsule (EC) and basal ganglia (BG). For statistics, a Mann Whitney test was used (significance: P<0.05).Results
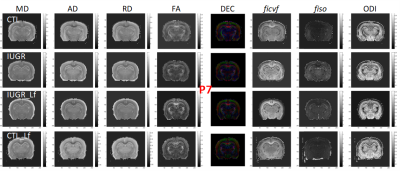
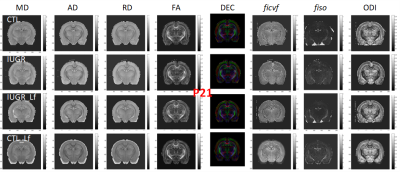
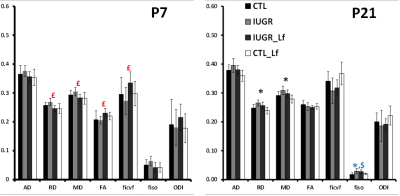
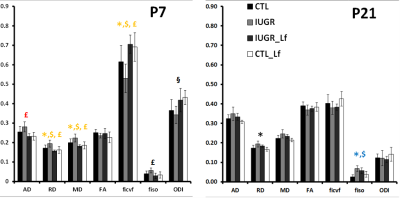
DTI and NODDI derived maps were of high quality with very good SNR at P7 (Fig. 1) and P21 (Fig. 2). In the Cx (Fig. 3), significant differences were observed at P7 between IUGR and IUGR_Lf, RD and MD were decreased whereas FA and ficvf were increased. In the other hand, IUGR_Lf values were not different from controls. At P21, caloric restriction induced significant increase of RD, MD and fiso, this last parameter was not restored by Lf. In the EC (Fig. 4), IUGR induced significant increase of RD and MD as well as decrease of ficvf partially restored by Lf at P7. At P21, RD and fiso remained significantly increased for IUGR compared to controls with poor effect of Lf. In the BG (Fig. 5), at P7 and P21 AD, RD and MD were significantly increased whereas fiso was significantly decreased following caloric restriction. Lf restored these parameters at the level of controls especially at P7. Effects of the CR were also visible on fiso and ODI which were not restored by Lf.Discussion and conclusion
In this study we characterized early and long-term effects of IUGR on brain development as well as neuroprotective effect of Lf. Our CR-induced IUGR model validity was confirmed. In the white and grey matter, microstructure disorganization was revealed following CR and partially restored by Lf.Acknowledgements
Supported by fond national Suisse n° 33CM30-124101/140334, the CIBM of the UNIL, UNIGE, HUG, CHUV, EPFL, Leenards and Jeantet foundation.References
[1] van de Looij Y et al. Cur. Opin. in Neur. 2014 Apr;27(2):157-67.
[2] van de Looij, Y., et al. (2014) Ann. Clin. Transl. Neurol. 1, 955–967.
[3] Ginet, V., et al. (2016) Biofactors 42, 323-336.
[4] Somm, E., et al. (2014) Pediatr. Res., 75, 51–61.
[5] Zhang H et al. (2012) Neuroimage Jul 16;61(4):1000-16.
Figures