2072
Differentiation and Quantification of White Matter Injury in Post-Hemorrhagic HydrocephalusAlbert M. Isaacs1, Harri Merisaari2, Tsen-Hsuan Abby Lin2, James (Pat) James McAllister3, David D Limbrick3, and Sheng-Kwei (Victor) Song2
1Neuroscience, Washington University School of Medicine, St. Louis, MO, United States, 2Radiology, Washington University School of Medicine, St. Louis, MO, United States, 3Neurosurgery, Washington University School of Medicine, St. Louis, MO, United States
Synopsis
This study is the first of its kind, and uses diffusion basis spectrum imaging (DBSI) to quantify, as well as differentiate the complex pathologies that underlies the white matter injury in post-hemorrhagic hydrocephalus (PHH) in neonates, using a ferret model of PHH.
Introduction:
Post-hemorrhagic hydrocephalus (PHH) is a debilitating neurological disorder, characterized by abnormal dilatation of the ventricles, following intraventricular hemorrhage (IVH). Up to 50% of preemie-IVH infants develop PHH, and suffer the worst neurologic outcomes in all newborn medicine, with cognitive deficits in 85% and cerebral palsy in 70% of cases. Most of PHH’s symptoms are attributed to periventricular white matter (PVWM) injury, and recent efforts have focused on Diffusion Tensor Imaging (DTI) as a biomarker. However, DTI has been shown to lack sensitivity and specificity for differentiating complex WM pathologies such as inflammation, edema, axonal injury, axonal loss and demyelination, which significantly limits its utility in PHH. We have utilized a novel multi-tensor technique, Diffusion Basis Spectrum Imaging (DBSI), which unlike DTI, can separate anisotropic from isotropic tensors. The DBSI anisotropic tensors model and resolve crossing fibers, and assess injured axons and demyelination. The isotropic tensor spectrum further categorizes restricted and non-restricted components, reflecting cellularity and edema respectively.Methods:
Autologous blood (n=7) or sham CSF (n=6) was injected into the ventricles of 20-day-old ferrets. Extracted brains at 50-day-old were scanned ex vivo in 4% PFA in an Agilent® 4.7 Tesla magnet. T2-Weighted images, and multi-echo spin echo diffusion weighted sequence in 99 diffusion directions (TR 3000 ms, TE 60 ms, Matrix = 192 x 192, b-value max = 3000 s/mm2) was acquired. The datasets were processed with in-house analysis package running on Matlab. Regions of Interest (ROIs) of PVWM and ventricular volume (VV) were delineated using ITK-SNAP. PVWM included: corpus callosum (CC) and anterior (ALIC) and posterior (PLIC) limbs of internal capsule. Voxel intensity values of all ROIs were extracted, and statistically analyzed using in-house Python v2.7 and R package v3.4.1 scripts. Parameter map (PM) intensity of ROIs, and VVs were analyzed with Shapiro-Wilk test for normality, non-paired t-tests for comparisons and 1-Way ANOVA for heteroskedastic data.Results
PHH was successfully induced (Figure 1). While the average VVs of the PHH group were larger than controls, it was not statistically significant (Figure 2). However, the PHH group had 68% (p<0.0004) proportional increase the hindered fraction, which included 120% (p=0.0006) and 51% (p=0.0005) in the CC and ALIC respectively (Figure 3). There was no significant change in PLIC. Assessing intrinsic PVWM injury, CC and ALIC sustained the most impact, demonstrating proportionally decreased fiber density by 7% (p=0.007) and 10% (p=0.03) respectively. ALIC also demonstrated evidence of axonal injury with decreased axial diffusivity of 8% (p=0.008), which is attributed in part to axonal loss, given the decrease in fiber fraction axial diffusivity of 9% (p=0.006). While similar trends were observed in the PLIC, none was statistically significant. Representative histological evidence of the PVWM inflammation is shown on Figure 4.Discussion:
PVWM injury is critical in PHH, and accounts for its most debilitating symptoms. Our findings agree with published literature that the corpus callosum (CC) and internal capsule are impacted the most. In our model, the hindered fractions of CC and ALIC of the PHH ferrets were doubled or increased by 50% respectively, when compared to controls. Given that there is no significant difference in their respective water fractions, the increase likely represents edema due to inflammation, induced by the IVH and/or progressive hydrocephalus. The finding of inflammation is also supported by the relatively high astrocytosis on histology in PHH than controls (Figure 4). We found similar evidence in postmortem brain specimen of human PHH. In humans, PVWM axonal integrity is also compromised in PHH. In our model, ALIC axons were most susceptible. In fact, over 7% of ALIC axons were lost, while 1 in every 10 fibers demonstrated axonal injury in the PHH group. Similar trends were seen in the CC and PLIC, but were not statistically significant.Conclusion
Our ferret PHH model mimics the human disease in four important ways: 1) evolution of PHH following IVH; 2) PVWM disruption in the CC and internal capsule; 3) WM injury occurs even when ventriculomegaly is not significantly apparent; 4) inflammation and edema plays a major role. DBSI is a versatile tool for differentiating and quantifying the different components of WM disruption in PHH. This experiment is the first of its kind to utilize any neuroimaging, specifically as a biomarker to differentiate and quantify PVWM inflammation from free water extravasation in hydrocephalus. Perhaps, more notably is DBSIs ability to differentiate PVWM axonal loss from injury. Further testing of DBSI in PHH both in vivo, and in humans is required to transition the modality from the bench to the bedside in PHH.Acknowledgements
Special thanks to all members of the Song and Limbrick lab for their support and help with this project.References
- Adams-Chapman I, Hansen NI, Stoll BJ, Higgins R, Network NR. Neurodevelopmental outcome of extremely low birth weight infants with posthemorrhagic hydrocephalus requiring shunt insertion. Pediatrics. 2008;121(5):e1167-1177.
- Shooman D, Portess H, Sparrow O. A review of the current treatment methods for posthaemorrhagic hydrocephalus of infants. Cerebrospinal Fluid Res. 2009;6:1.
- Juan W, McKinstry RC, Shimony JS, et al. Diffusion tensor imaging properties and neurobehavioral outcomes in children with hydrocephalus. AJNR Am J Neuroradiol. 2013;34(2):439-445.
- Wang X, Cusick MF, Wang Y, et al. Diffusion basis spectrum imaging detects and distinguishes coexisting subclinical inflammation, demyelination and axonal injury in experimental autoimmune encephalomyelitis mice. NMR Biomed. 2014;27(7):843-852.
- Wang Y, Wang Q, Haldar JP, et al. Quantification of increased cellularity during inflammatory demyelination. Brain. 2011;134(Pt 12):3590-3601.
- Ahn SY, Chang YS, Sung DK, et al. Mesenchymal stem cells prevent hydrocephalus after severe intraventricular hemorrhage. Stroke. 2013;44(2):497-504.
- McAllister JP, Guerra MM, Ruiz LC, et al. Ventricular Zone Disruption in Human Neonates With Intraventricular Hemorrhage. J Neuropathol Exp Neurol. 2017;76(5):358-375.
- Del Bigio MR. Neuropathology and structural changes in hydrocephalus. Dev Disabil Res Rev. 2010;16(1):16-22.
Figures
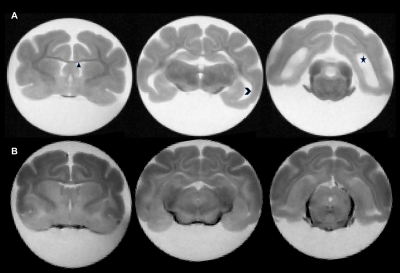
Figure 1: Representative ex vivo trans-axial T2W images of two 50-day old
ferrets. Panels A and B demonstrate the presence and absence
of PHH in the IVH-induced and Sham Control ferrets respectively. Hydrocephalus
is demonstrated by the significantly enlarged frontal (diamond), temporal
(chevron) and occipital horns of the lateral ventricles of the PHH ferret (A)
when compared to non-PHH ferret (B).
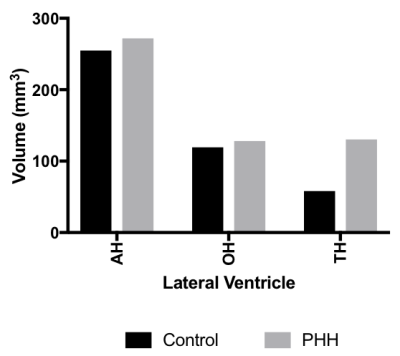
Figure 2: Ventricular volumes calculated from ROIs
demarcating sections of the lateral ventricles demarcated as: Frontal (AH),
occipital (OH) and temporal (TH) horns
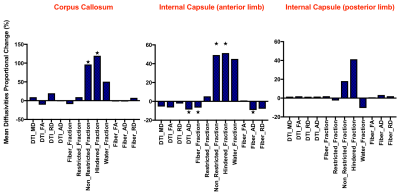
Figure 3: Proportional change in mean diffusivities of PHH from control ferrets
in PVWM
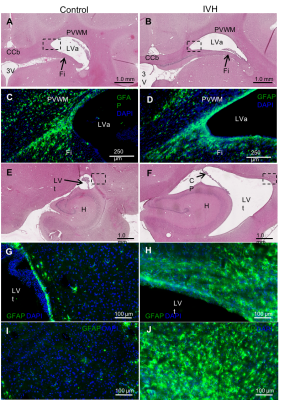
Figure 4: Representative
photomicrographs comparing ventricular size and astrocyte responses in sham
control (A, C, E, G, I) and IVH (B, D, F, H, J) ferrets. The number and
reactive cytology of GFAP-positive astrocytes in the ventricular zone (G&H)
and the PVWM lateral and dorsal to the temporal horn of the lateral ventricle, LVt
(I&J) are considerably higher in IVH. Additional abbreviations: LVa-Atrium
of the lateral ventricle CCb–body of the
CC; CP–choroid plexus; DAPI
-4,6-Diamidino-2-phenylindole, dihydrochloride immunostaining; GFAP –glial
fibrillary acidic protein; H–hippocampus; 3V–third ventricle.