2061
Revealing the three-dimensional intraparenchymal trajectory of the brainstem cranial nerve systems by diffusion MRI representation.1QMI/NIBIB, National Institutes of Health, Bethesda, MD, United States, 2Henry M. Jackson Foundation, Bethesda, MD, United States, 3NINDS, National Institutes of Health, Bethesda, MD, United States, 4NCI, National Institutes of Health, Bethesda, MD, United States, 5NHGRI, National Institutes of Health, Bethesda, MD, United States
Synopsis
The cranial nerve systems of the human brainstem are challenging to distinguish from their complex architectural surroundings, but anisotropy, orientation and tract-based diffusion MRI methods may address these challenges and enable mapping intraparenchymal trajectories of the cranial nerves. The objective of this study was to apply and evaluate DTI and tractography tools for segmentation and mapping of the cranial nerve systems at high spatial resolution in post-mortem human brainstems. Our findings demonstrate the salient features of scalar, directional and tract-based maps for distinguishing the cranial nerves and their nuclei with attention to their relative geometric complexity and architectural environment.
Introduction
Brainstem neuroanatomy is remarkably complex, with a compact arrangement of gray matter nuclei, white matter tracts and nerve fibers that are diverse in their functions, geometric features and anatomic connections1. Non-invasive mapping of the brainstem is not yet adequate to resolve key neuroanatomical features due to the sub-milimeter scale of many brainstem structures and the homogeneous tissue contrast across tracts and fibers. If MRI methods can be developed to accomplish this, it would improve pre-surgical planning for brainstem surgery and for the cranial nerve systems in particular would benefit the basic understanding of cranial nerve disorders by revealing the presence and trajectory of abnormal nerves, which are currently unknown. With these goals in mind, we have applied high-resolution diffusion MRI with comprehensive DWI sampling to generate annotated DTI maps, ROI segmentations and tractography for each cranial nerve system within post-mortem brainstem specimens and examined the adequacy of these methods to map the intraparenchymal trajectory of cranial nerves.Methods
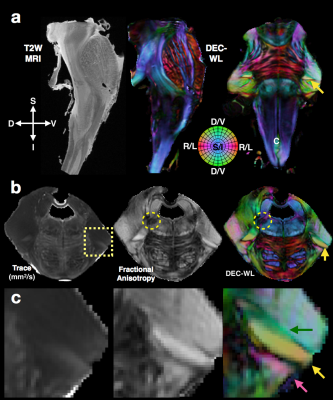
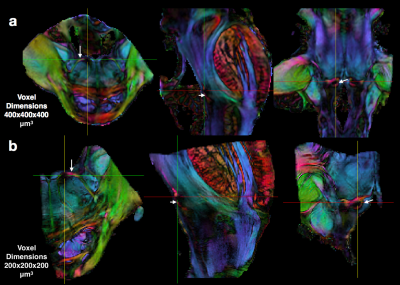
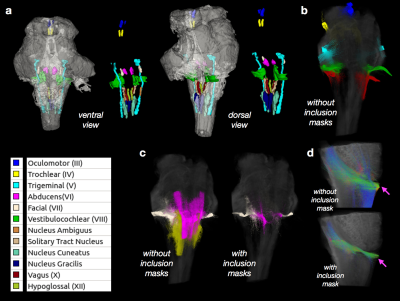
Two brainstem specimens having no expected abnormalities were obtained from the NIH pathology department and imaged using a 4.7T MRI scanner to acquire DWI volumes with 400 micron isotropic resolution and then partial specimens of the left ponto-medullary junction were prepared and imaged using a 7T or 14T MRI scanner to acquire DWI volumes with 200 micron isotropic resolution. For all DWIs a 3D-EPI pulse sequence was used to collect 243-297 DWIs with a multi-shell sampling scheme with b=250-10,000 s/mm2. Structural MRI scans were also obtained with similar spatial dimensions as the DWI volumes. Total scan time for each specimen was between 50 and 100 hours. Corrections and other processing of the DWI data and DTI fitting was performed using TORTOISE3 software2, which generated scalar DTI and directionally encoded color (DEC) maps3. DEC maps weighted by linear anisotropy4 (DEC-WL) provided the best visualization of brainstem fibers and tracts and are used in all figures. Constrained spherical deconvolution, tractography and tract-weighted imaging (TWI)5 was performed with mrtrix3 software6 using DWI data from a single shell (b=6000 or 10000, 87 directions) and an unweighted image. The nuclei of the cranial nerves were segmented based on several DTI maps using ITKsnap software and tract representations of the cranial nerves were generated in mrtrix3 software using seed regions placed within the nerve roots and when indicated inclusion masks placed near the nuclei.Results
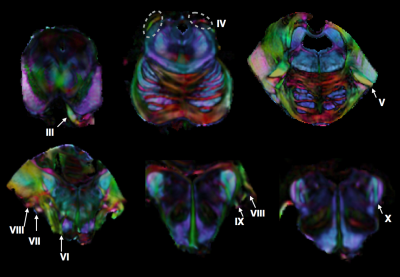
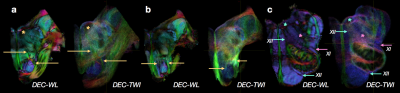
High-quality DTI maps of scalar values and directional information were found to distinguish different features of brainstem neuroanatomy (Figure 1) and the high-resolution 3D acquisition used in this study enabled continuous visualization of small fiber systems with complex trajectories (e.g. VII, Figure 2). Most nerve roots were readily identifiable using DEC-WL maps (Figure 3) and the segmentation of brainstem nuclei (Figure 4a) was accomplished using the DTI scalar maps, especially by FA contrast fro which values within the nucleus were low compared with surrounding tissue (yellow circle, Figure 1). Tractography with seed regions of the nerve roots resulted in neuroanatomically reasonable representations for nerves III, IV, V, VIII and IX (Figure 4b), but for nerves VI, VII, XI and XII few fiber tracts reached their known nuclei connection and many tracts erroneously joined adjacent, but functionally unrelated fiber pathways (Figure 4c,d). Exploration of the nerve trajectories for VI and XII by DEC-WL and TWI maps in the partial specimen demonstrated the small diameter, multi-fiber anatomy and complexity of the tissue environment of the nerve as major challenges to full tract representation, although portions of the intraparenchymal trajectories for these nerves were evident (Figure 5).Discussion and Conclusions
In this study, DTI and tract-based representation of ex-vivo human brainstems revealed local anatomical details of each cranial nerve system that were most evident for maps using directional information and linear anisotropy. Salient features of the DTI scalar maps were sufficient for coarse segmentation of the brainstem nuclei, but the major strength of DTI for cranial nerve representation was the ability to distinguish cranial nerves from their complex architectural surroundings in many cases. However, the limitations of these approaches were also evident given the incomplete trajectory representation for nerve systems with ventral emergence having small fibers and traversing major tracts especially in the dorsal brainstem. Despite the remarkable complexity of the human brainstem and small diameter of the cranial nerves, DTI maps and tract-based representation were able to reveal considerable detail of the fiber geometry, 3D spatial organization and intraparenchymal trajectory of these nerve systems. The advantages and limitations reported for mapping cranial nerve systems in the human brainstem can advance the use of these methods for research and clinical applications.Acknowledgements
The authors thank the Department of Pathology for providing whole brainstem specimens, Dr. Ray-Chaudhry for dissection and the Mouse Imaging Facility for assistance with MRI.References
1. Naidich TP, Duvernoy HM, Delman BN, Sorensen AG. Duvernoy's atlas of the human brain stem and cerebellum: high-field MRI, surface anatomy, internal structure, vascularization and 3 D sectional anatomy. Duvernoy's atlas of the human brain stem and cerebellum: high-field MRI, surface anatomy, internal structure, vascularization and 3 D sectional anatomy 2009.
2. Pierpaoli C, Walker L, Irfanoglu M, Barnett A, Basser P, Chang L, Koay C, Pajevic S, Rohde G, Sarlls J. TORTOISE: an integrated software package for processing of diffusion MRI data. Book TORTOISE: an Integrated Software Package for Processing of Diffusion MRI Data (Editor ed^ eds) 2010;18:1597.
3. Pajevic S, Pierpaoli C. Color schemes to represent the orientation of anisotropic tissues from diffusion tensor data: Application to white matter fiber tract mapping in the human brain. Magnetic Resonance in Medicine 1999;42(3):526-540.
4. Westin CFF, Maier SE, Mamata H, Nabavi A, Jolesz FA, Kikinis R. Processing and visualization for diffusion tensor MRI. Medical image analysis 2002;6(2):93-108.
5. Calamante F, Tournier J-DD, Jackson GD, Connelly A. Track-density imaging (TDI): super-resolution white matter imaging using whole-brain track-density mapping. NeuroImage 2010;53(4):1233-1243.
6. Tournier J, Calamante F, Connelly A. MRtrix: diffusion tractography in crossing fiber regions. International Journal of Imaging Systems and Technology 2012;22(1):53-66.
Figures