2056
Regional Brain Iron Mapping in Patients with Heart Failure1UCLA School of Nursing, University of California at Los Angeles, Los Angeles, CA, United States, 2Department of Anesthesiology, University of California at Los Angeles, Los Angeles, CA, United States, 3Division of Cardiology, University of California at Los Angeles, Los Angeles, CA, United States, 4Department of Radiological Sciences, University of California at Los Angeles, Los Angeles, CA, United States, 5Department of Bioengineering, University of California at Los Angeles, Los Angeles, CA, United States, 6Brain Research Institute, University of California at Los Angeles, Los Angeles, CA, United States
Synopsis
HF subjects show brain injury in multiple areas, which may contribute to altered iron concentration in those sites. However, regional brain iron load in HF subjects is unclear. We examined regional iron deposition using R2*-relaxometry procedures and found altered R2*-values in the amygdala, brainstem, thalamus, globus pallidus, hippocampus, cerebellum, insula, and frontal and temporal white matter regions. The altered iron concentration in HF subjects may result from neural and white matter injury, including myelin and glial dysfunction, with iron potentially accelerating tissue degeneration. These data suggest that interfering with the iron action may reduce the exacerbation of injury in HF.
Purpose
Heart failure (HF), a common cardiovascular condition, shows widespread brain injury in both gray and white matter regions that are responsible for autonomic, respiratory, mood, and cognition control.1,2 Brain tissue injury, including dysfunctional myelin and glial cells, as well as axonal loss are commonly observed in the condition that may contribute to altered iron concentration in those sites. Iron is an essential component for synthesis of neurotransmitters and myelin function; surplus iron concentration can create free radicals and elevate oxidative stress, leading to neurodegeneration on the surrounding tissue,3 and deficit of iron can affect brain glial and myelin functions.4 However, the levels and distribution of regional brain iron load in HF subjects is unclear. Brain iron content can be assessed non-invasively by R2* (1/T2*) relaxation rate procedures, and have been used to evaluate iron levels in various conditions with variable iron loads with disease severity and disease duration,5,6 and thus, may be useful to examine HF subjects. Our aim was to examine regional brain iron deposition in HF, compared to control subjects using R2*-relaxometry procedures. We hypothesized that R2*-relaxometry procedures will show altered regional iron deposition in multiple brain sites in HF over control subjects.Theory
The R2* maps from the multiple echo T2*weighted images can be estimated by employing the following equation: R2* = $$=-\frac{\sum_n^Nlog(\frac{I_{n}}{I_{1}})*(TE_{n}-TE_1)}{\sum_n^N(TE_{n}-TE_{1})^{2}}$$Where N is number of echo times, In is T2*-weighted image at nth echo time and I1 is signal intensity of T2*-weighted image at first echo time, n= 2, 3, 4.Materials and Methods
We examined 20 HF (age, 52.9±7.4 years; body-mass-index (BMI), 27.5±5.3 kg/m2; 13 male; NYHA functional class II/III, LVEF <40) and 69 control subjects (age, 51.0±8.8 years; BMI, 26.5±3.6 kg/m2; 44 male). Control subjects were healthy, without any medications that might alter brain tissue. Brain imaging studies were performed using a 3.0-Tesla MRI scanner (Magnetom Prisma; Siemens). T2*-weighted imaging was performed using a gradient-echo pulse sequence with multiple echo times (TR = 1800 ms; TEs = 5, 12, 20, and 30 ms; flip-angle = 18°; matrix-size = 256×256; FOV = 230×230 mm; slice-thickness = 3.6 mm). High-resolution T1-weighted images were collected using a MPRAGE pulse sequence (TR = 2200 ms; TE = 2.4 ms; inversion-time=900 ms; FA = 9°; matrix size = 320×320; FOV = 230×230 mm2; slice thickness = 0.9 mm). Using T2*-weighted images from different TEs, R2*maps were calculated voxel-by-voxel with multi-exponential curve fitting, as described above. The R2* maps were normalized to Montreal Neurological Institute (MNI) space, using unified segmentation, and smoothed (Gaussian kernel, 8 mm). High-resolution T1-weighted images of control subjects were also normalized to MNI space for background images. The smoothed R2* maps were compared voxel-by-voxel between groups using ANCOVA (covariates: age, gender; SPM12, uncorrected threshold p < 0.005). Brain clusters with significant differences between groups were overlaid onto background images for structural identification.Results
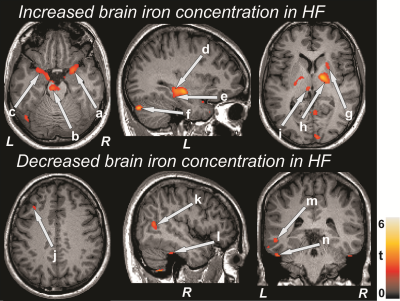
No significant differences in age (p = 0.39), gender (p = 0.92), or BMI (p = 0.43) appeared between groups. Multiple brain areas in HF showed increased R2* values, but few regions appeared with decreased R2* values in HF over control subjects (Fig. 1). Brain sites that showed increased R2* values in HF subjects included the bilateral amygdala (Fig 1a, c), bilateral pons (Fig 1b), bilateral hippocampus (Fig 1d), left para-hippocampal gyrus (Fig 1e), bilateral medulla, right posterior cingulate, left cerebellum (Fig 1f), bilateral insula, bilateral prefrontal cortices, bilateral frontal white matter, right putamen (Fig 1g), bilateral globus pallidus extending to internal capsule (Fig 1h), and bilateral thalamus (Fig 1i). Regions with decreased R2* values in HF included the bilateral frontal cortices (Fig 1j), right superior (Fig 1k) and left middle temporal gyrus (Fig 1m), left superior temporal white matter, and bilateral inferior temporal gyrus (Fig 1l, n).Discussion
Quantitative assessment by R2*-relaxometry suggests altered regional brain iron deposition in HF over control subjects. The altered iron content appeared in both brain gray and white matter areas that showed tissue injury previously. The pathological mechanisms for excess iron deposition in HF subjects may include accumulation from neural and white matter injury, including myelin and glial dysfunction, and may accelerate tissue degeneration in the condition. The iron deficiency, as observed in few brain regions, might alter several brain proteins, such as dopamine D2 receptors whose inefficient interaction with opiate peptides has detrimental effect in learning and cognition, as evident in the condition. These findings may suggest means for intervention to lessen neural injury by interfering with the iron processes exacerbating the damage in HF subjects.Conclusion
Our study indicates the presence of altered regional brain iron concentration in HF subjects.Acknowledgements
This work was supported by National Institutes of Health R01 NR-013625, R01 NR-014669, and American Heart Association 17POST33440099 (B.R.).References
1. Woo MA, Kumar R, Macey PM, et al. Brain injury in autonomic, emotional, and cognitive regulatory areas in patients with heart failure. J Card Fail. 2009;15:214-223.
2. Kumar R, Woo MA, Macey PM, et al. Brain axonal and myelin evaluation in heart failure. J Neurol Sci. 2011;307:106-113.
3. Andrews NC. Disorders of iron metabolism. The New England journal of medicine. 1999;341:1986-1995.
4. Youdim MB. Brain iron deficiency and excess; cognitive impairment and neurodegeneration with involvement of striatum and hippocampus. Neurotox Res. 2008;14:45-56.
5. Roy B, Verma S, Awasthi R, et al. Correlation of phase values with CT Hounsfield and R2* values in calcified neurocysticercosis. J Magn Reson Imaging. 2011;34:1060-1064.
6. Khalil M, Enzinger C, Langkammer C, et al. Quantitative assessment of brain iron by R(2)* relaxometry in patients with clinically isolated syndrome and relapsing-remitting multiple sclerosis. Mult Scler. 2009;15:1048-1054.